Introduction
Cardiovascular disease is the primary cause of global mortality, with cardiovascular disease-related deaths surging from 17 million in 2008 to 25 million by 2020 (Zahra et al., 2015). Prevention and management are paramount due to cardiovascular disease’s substantial social and economic burdens. Noteworthy risk factors for cardiovascular disease encompass high blood pressure, elevated total cholesterol, triglycerides, and LDL-c, reduced HDL-c levels, insulin resistance, hypertension (HT), and type 2 diabetes mellitus (Virani et al., 2020). Adopting a healthy diet is a pivotal strategy for mitigating these cardiovascular disease risk factors (Virani et al., 2020). The consumption of fruits and vegetables has been linked to reductions in blood pressure (Siervo et al., 2013) and enhancements in endothelial function (Lara et al., 2016).
Lycopene, a natural pigment found in tomatoes, watermelon, pink grapefruit, papaya, guava, and rosehip, boasts a molecular structure featuring eleven conjugated double bonds, rendering it a potent antioxidant and scavenger of free radicals (Riccioni et al., 2008). Lycopene is the primary carotenoid in human plasma, effectively shielding the body from oxidative stress (Riccioni et al., 2008). Clinical trials have demonstrated the significance of lycopene in reducing OS, thereby preventing the oxidation of LDL-c (Basu and Imrhan, 2007;Riccioni et al., 2008). The oxidation of LDL particles triggers inflammatory processes, giving rise to foam cell formation, fatty streaks, atherosclerotic plaques, and plaque rupture (Riccioni et al., 2008). ox-LDL particles impede normal endothelial function by inhibiting the release of nitric oxide (NO), a critical vasodilator, consequently influencing blood pressure (Willcox et al., 2003).
The hypothesis underpinning this systematic review is that lycopene may exert favorable effects on cardiovascular risk factors through its antioxidative properties and potential impact on inflammatory pathways. By compiling and analyzing data from randomized-controlled trials, this review aims to elucidate whether lycopene interventions significantly improve blood pressure, blood lipid profiles, and inflammation and oxidative stress markers in patient populations.
Materials and Methods
This systematic review was conducted by Cochrane guidelines (Higgins et al., 2019) and adhesive to PRISMA guidelines (Moher et al., 2009). The investigation period spanned from October 2020 to April 2021, during which we systematically searched four electronic databases from their inception: Web of Science, Embase, and Cochrane. The search terms employed encompassed both lycopene and relevant medical subject headings descriptors in conjunction with terms associated with cardiovascular disease (including cardiovascular or CVD, coronary, heart defect, heart failure, heart disease, arrhythmia, hypertension, hypotension, myocardial infarction, infarction, atherosclerosis, ischemia, cardiac, or stroke).
For inclusion in this study, the following criteria were applied: (1) study design: randomized-controlled trials; (2) participants: adults aged 19 years and older; (3) interventions: lycopene interventions, whether dietary or supplementary; (4) outcomes: measures related to cardiovascular disease, including at least one cardiovascular risk factor such as blood pressure, blood lipid levels, inflammatory markers, or oxidative stress markers. Exclusion criteria were as follows: (1) study design: non-interventional studies; (2) participants: individuals under the age of 19 years; (3) interventions: studies not involving lycopene, interventions that combined multiple substances, making it impossible to isolate the effects of lycopene, or interventions where the dose of lycopene could not be determined; (4) outcomes: measures unrelated to vascular health. Two independent researchers conducted assessments to determine eligibility. Initially, studies were assessed based on their titles and abstracts. In cases of uncertainty, the study was included for further evaluation. Subsequently, selected articles underwent full-text screening, and any conflicts were resolved through consensus.
A standardized template was employed to gather pertinent information from the 13 incorporated studies to evaluate study quality and evidence analysis. The information extracted encompassed participant characteristics (comprising health status, age, and the number of participants in both treated and control groups), study design, the method of intervention (either dietary or via supplements), the duration of the intervention, the dosage of lycopene administered, the control method (involving diet or placebo), and the outcomes observed (including whether significant changes occurred or if there was no change).
According to Cochrane’s protocol, two independent researchers assessed all eligible articles’ quality. The evaluation was done using the Cochrane ‘Risk of Bias’ tool. Per our predetermined criteria, the studies were categorized into three quality levels: low, high, or some concern.
Results and Discussions
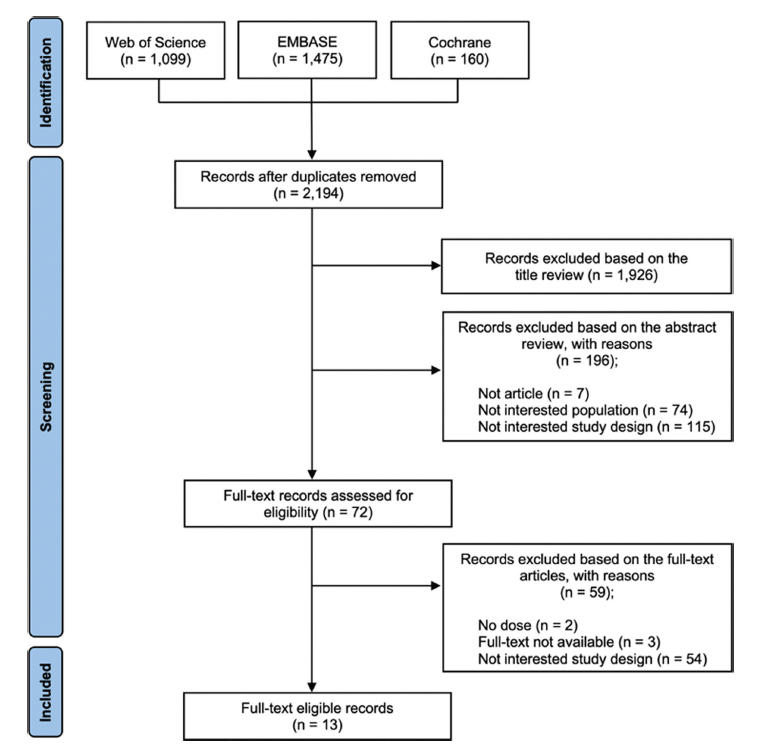
A total of 2,734 articles were identified from the database searches. After duplicates were excluded, 2,194 articles remained and were assessed for eligibility based on titles and abstracts. Through title screening, 1,926 papers were excluded, leaving 268 papers. Subsequently, abstract review was conducted, evaluating study design, population, full text, and English articles, resulting in the exclusion of 196 papers, leaving 72 papers. Studies were included at this stage only if the study design, interventions, and outcome meet the inclusion criteria: randomized-controlled trials, patient subjects, lycopene intervention, clear dosage, duration, and outcome in English, and full-text available. Further full-text review was performed, assessing aspects like study design and specific dosage, resulting in the exclusion of 59 papers, 13 studies were finally included in this review. Fig. 1 shows the inclusion process.
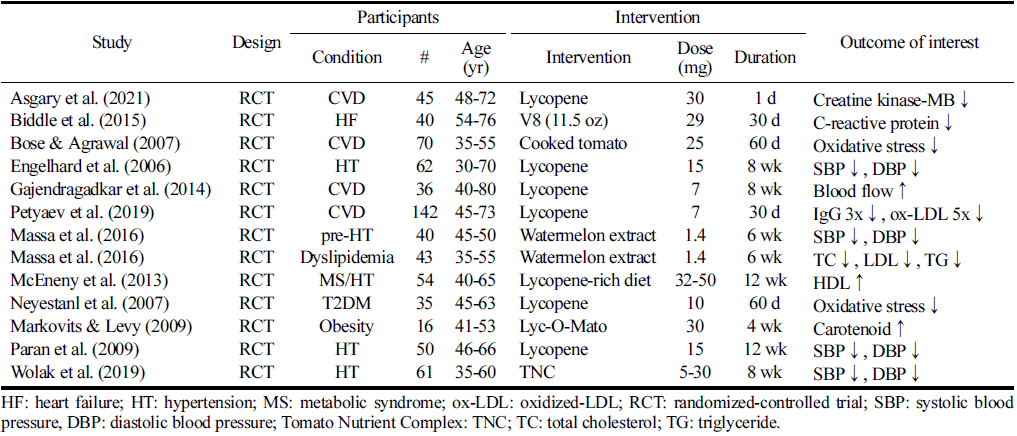
In the 13 articles incorporated into this analysis, lycopene interventions encompassed a cohort of 385 male and female participants, all exhibiting at least one cardiovascular disease risk factor. The age range of the participants spanned from 8 to 74 years. Eight of these studies involved lycopene interventions compared to a placebo control group (Bose et al., 2007;McEneny et al., 2013;Biddle et al., 2015). Three studies utilized a usual diet-controlled group (Bose et al., 2007;McEneny et al., 2013;Biddle et al., 2015), while one study employed a lactolycopene-controlled group (Petyaev et al., 2019), and another study utilized a control group receiving standard treatment, either statin or aspirin (Asgary et al., 2021). Additionally, one study adopted a cross-over study design (Paran et al., 2009) in their investigation.
In the referenced manuscript, researchers employed various approaches to introduce lycopene into their studies. These methods included incorporating it into the participants’ diets or providing lycopene supplements. Of the investigated studies, eight opted for lycopene supplementation, three utilized a dietary approach incorporating tomato-based products, and two employed watermelon extracts. The amount of lycopene administered varied across the studies, ranging from 1.44 to 50 mg of lycopene per day, with an average dosage of 19.2 mg per day. Five studies implemented interventions involving a lycopene dosage equal to or exceeding 19.2 mg per day. Additionally, the duration of lycopene intervention spanned from a single day to a maximum of 12 weeks.
Lycopene was delivered as food in two studies out of a total of 13 studies. It is difficult to differentiate the effects between lycopene and other nutrients in the diet. Another difficulty is the baseline or habitual levels of lycopene in a subject’s diet. Some studies attempt to control this by having a wash-out period or a low-lycopene diet; however, this is inconsistent across studies. The dose of lycopene varied (1.44 to 50 mg/d) with different durations (1 day to 12 weeks), making it difficult to establish the optimal dosage.
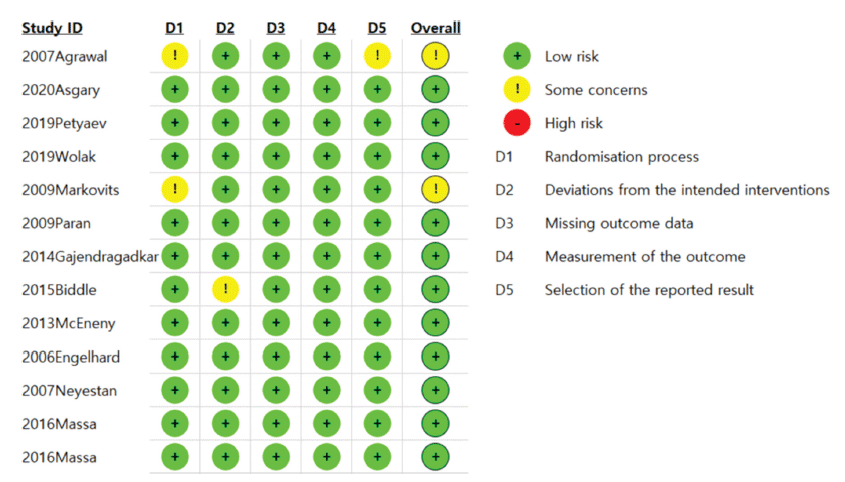
All the studies in the analysis met the quality assessment criteria, encompassing aspects such as randomization, the intended intervention, measurement of outcomes, and selection of reported results. Eleven studies were classified as ‘low’ quality, while two fell into the ‘some concern’ category due to insufficient details regarding the randomization method and non-randomized allocation (Fig. 2).
Five studies reported blood pressure as an outcome measure, and the data extracted from these articles are shown in Table 1. Three studies reported statistically significant decreases in blood pressure compared with a control group (Engelhard et al., 2006;Paran et al., 2009;Massa et al., 2016a;Wolak et al., 2019), whereas one reported no effect (Gajendragadkar et al., 2014). Five studies that significantly improved blood pressure used a supplementary intervention. The largest significant effects were with hypertensive participants. Systolic blood pressure decreased by 11.8m mHg in the watermelon group, producing final mean values of 126.0±4.0 mmHg, and diastolic blood pressure decreased by 6.9 mmHg, producing a final mean of 72.3±2.0 mmHg (Massa et al., 2016a). Similarly, there was a decrease in diastolic blood pressure from 82.1±7.2 to 77.9±6.8m mHg (p<0.001) and from 80.1±7.9 to 74.2±8.5 mmHg (p<0.001) (Paran et al., 2009). In a dose-response investigation involving lycopene intervention at 5, 15, and 30 mg doses, as compared to a placebo-controlled group, it was observed that a significant improvement in systolic blood pressure occurred at lycopene doses of 15 mg or higher (Wolak et al., 2019).
|
Similarly, other studies show that controlling blood pressure reduces cardiovascular events and death (Hansson et al., 1998;Estacio et al., 2000;Düsing, 2016). While these findings suggest a potential benefit of lycopene in controlling blood pressure, it remains challenging to determine the optimal lycopene intake for managing blood pressure effectively. Therefore, it is imperative for future research to conduct welldesigned studies exploring the precise dosage and long-term effects of lycopene supplementation on blood pressure regulation to provide more definitive guidance on its role in achieving effective blood pressure control.
The effects of lycopene on blood lipids were reported in seven studies (Table 1). Two studies found significant improvements in the effect of lycopene on ≥1 measure of blood lipids (McEneny et al., 2013; Massa et al., 2016b), while other studies showed no significant change in patient subjects (Engelhard et al., 2006;Bose et al., 2007;Markovits & Levy, 2009;Gajendragadkar et al., 2014;Petyaev et al., 2019). Significant reductions in TC and LDL-c, observing a mean decrease of TG by 38.05 mg/dL in the experimental group compared to an increase of 2.6 mg/dL in the control group (p<0.001) with 6 g watermelon extract (1.44 mg lycopene) for 6 weeks in metabolic syndrome patients (Massa et al., 2016b). A significant increase in HDL (p<0.01) with dietary lycopene intake delivered as a lycopene supplement or with lycopenerich foods (McEneny et al., 2013).
Moreover, it is worth noting that although a growing body of research highlights the positive impact of lycopene on risk factors associated with cardiovascular disease, our present systematic review excludes this study due to the need for more information regarding the dosage used. Nonetheless, several studies have demonstrated encouraging results in this regard. For instance, there was a significant reduction in TC and LDLc in ultra-marathon runners with tomato consumption for 2 months (Samaras et al., 2014). An 8.2% increase in HDL-c (p<0.05) and a significant decrease of ≤14.5% in LDL-c (p<0.001) with tomato juice for 2 months with subjects of metabolic syndrome (Tsitsimpikou et al., 2014). However, due to the no information on dosage, this study is excluded from the current systematic review.
Table 1 shows a comprehensive overview of the individual studies' findings regarding the impact of lycopene on oxidative stress and its potential anti-inflammatory effects. Five studies reported that lycopene interventions resulted in statistically significant improvements in ≥1 oxidative stress or inflammatory markers compared to the control group (Bose et al., 2007;Neyestani et al., 2007;McEneny et al., 2013;Biddle et al., 2015;Petyaev et al., 2019). Two studies (Bose et al., 2007;Neyestani et al., 2007) demonstrated significant reductions in malondialdehyde, which stimulates pro-atherogenic specific T cell-dependent response and specific antibody formation, mostly of IgG isotype. The reduction of malondialdehyde [0.6 nmol/mL (p<0.05), -0.439 nmol/h (p<0.001), respectively] highlights lycopene's potential in mitigating oxidative stressinduced damage. In addition, a lycopene dose of 29.4 mg/d for 30 days in patients with heart failure significantly reduced Creactive protein concentrations in women but not men (Biddle et al., 2015). Lycopene prevents post-PCI myocardial damage by inhibiting the increase of creatine kinase-MB (Asgary et al., 2021). These results indicate the potential role of lycopene in mitigating oxidative stress-induced damage and reducing inflammation.
Conclusions
We examined the impact of lycopene on various cardiovascular health markers across a selection of 13 studies. These investigations collectively comprised 385 participants, all exhibiting at least one cardiovascular disease risk factor, providing valuable insights into the potential benefits of lycopene supplementation or dietary incorporation. Notably, the most substantial reductions were observed in hypertensive participants, with significant systolic and diastolic blood pressure decreases. These findings are particularly promising given the well-established connection between blood pressure control and reduced cardiovascular events and mortality. Another critical aspect examined was the impact of lycopene on blood lipid profiles, which suggests that lycopene may have a positive effect on blood lipid levels. However, further research is needed to establish these outcomes’ consistency and determine optimal dosages and durations. Additionally, the studies reviewed shed light on the potential of lycopene to mitigate oxidative stress and inflammation.
While the findings of this study hold promise for the potential benefits of lycopene in managing cardiovascular disease risk factors such as blood pressure, lipid profiles, oxidative stress, and inflammation, it is crucial to acknowledge the study’s limitations. Variations in lycopene dosage and duration among the examined studies pose challenges in determining the optimal regimen for achieving desired health benefits. Furthermore, studies that incorporated lycopene-rich foods face the complexity of distinguishing lycopene’s effects from those of other nutrients present in the diet. There is a pressing need for further well-designed studies that systematically investigate the most effective dosages, durations, and delivery methods. These future studies can help refine our understanding of lycopene’s potential and its precise mechanisms in promoting cardiovascular well-being. Nevertheless, these findings underscore the promising potential of lycopene, whether obtained through dietary sources or supplements, as an intervention strategy for improving cardiovascular health.







