Introduction
Recent advances in the study of gut microbiota have led to the emergence of probiotics and prebiotics as functional foods. The gut microbiota, which maintains immune and metabolic homeostasis, is a community of microorganisms that inhabit the gastrointestinal tract of humans and animals (Sekirov et al., 2010). Recently, probiotics and prebiotics have been intensively studied on the modulation of the gut microbiota more positively. The control of the gut microbiota by probiotics helps treat many diseases (Floch & Montrose, 2005), and probiotics have been shown to maintain gut microbiota homeostasis (Larsen et al., 2010;Ejtahed, Mohtadi-Nia et al., 2012).
Gibson et al. (1995) defined prebiotics as “nondigestible substances that are selectively fermented by intestinal microbes and affect host health. Prebiotics are generally synthesized using glucose, galactose, xylose, and fructose as substrates, and commercially available major prebiotics include fructo-oligosaccharides (FOS), inulin, and galactooligosaccharides (Fuller & Gibson, 1998). Also, fruits, vegetables, cereals, and other edible plants are carbohydrate sources constituting potential prebiotics (Markowiak & Śliżewska 2017). The benefit of prebiotics to the human body is that prebiotics are fermented into short-chain fatty acids by probiotic strains in the gut and lower the pH of the colon and inhibit the growth of pathogens (Jayachandran et al., 2017).
Nowadays, interest in novel prebiotic substances is increasing, and many researchers are conducting many studies on whether oligosaccharides produced by lactic acid bacteria (LAB) are effective as prebiotic substances. Glycosyltransferase (EC 2.4.5.1; also called glucansucrase) can be classified into dextransucrase (synthesizes α-1,6 glycosidic bonds), mutansucrase (synthesizes α-1,3 glycosidic bonds), alternansucrase (synthesizes α-1,3 and α-1,6 alternating glycosidic bonds), and reuteransucrase (synthesizes α-1,4 glycosidic bonds) according to the structure of the synthesized gluco-oligosaccharides (Leemhuis et al., 2013). Glycosyltransferases are extracellular enzymes mainly produced by LAB, such as Weissella, Lactobacillus, and Leuconostoc spp. In this study, Weissella cibaria YRK005, which produces oligosaccharides in good yield, was isolated from young radish kimchi, and the structure and potential prebiotic effects of oligosaccharides from W. cibaria YRK005 were investigated.
Materials and Methods
Oligosaccharide-producing LAB were isolated from young radish. Ten grams of young radish were suspended in 100 mL sterilized saline [0.88% (w/v) NaCl], and the resuspension was spread on phenylethyl alcohol agar plate (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) containing 2% (w/v) sucrose (PES agar plate) and incubated at 30°C for 48 h. Viscous colonies were selected, and pure culture was performed on plate count agar (1 g dextrose, 5 g tryptone, 2.5 g yeast extract, and 15 g agar, per liter) with 0.6% bromocresol purple.
To identify the selected LAB strain, genomic DNA isolation, nucleotide sequencing of the 16S rRNA gene, and phylogenetic analysis were carried out as described previously (Lee et al., 2018a). Scanning electron microscopy (SEM) was performed using field-emission SEM (Zeiss SUPRA 66VP, Carl Zeiss Microscopy GmbH, Jena, Germany; installed at the National Instrumentation Center for Environmental Management, Seoul National University, Seoul, Korea).
Oligosaccharides were produced by W. cibaria YRK005 using LM broth (2 g peptone, 4 g yeast extract, 0.02 g NaCl, 2 g potassium phosphate, 0.4 g magnesium sulfate, 0.02 g ferrous sulfate, 0.02 g manganese (II) sulfate, 0.03 g calcium chloride, 147 g sucrose, and 135 g maltose, per liter) by incubating at 30°C at appropriate times. The culture supernatant was concentrated 15-fold at 80°C using a rotary evaporator, and the concentrate was loaded onto Bio-gel P2 resin (Bio-Rad Laboratories, Inc., Hercules, CA, USA) packed in a glass Econo-column (1.5×120 cm; Bio-Rad Laboratories). The elutes were collected using a fraction collector (Gilson, Inc., Middleton, WI, USA) with a fraction volume of 5 mL/tube at a flow rate of 30 mL/h. The oligosaccharide fractions analyzed by thin-layer chromatography (TLC) were pooled and freezedried (Kim et al., 2021).
TLC and high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), sizeexclusion high-performance liquid chromatography (HPLC), 1H-nuclear magnetic resonance (NMR) spectroscopy, and monosaccharide composition and glycosidic bond composition analyses were performed as described previously (Kim et al., 2021). Briefly, TLC was performed using silica gel 60F254 (Merck, Darmstadt, Germany), and TLC plate was developed using a solvent consisting of nitromethane (Sigma-Aldrich, St. Louis, MO, USA)/n-propyl alcohol (Samchun, Seoul, Korea)/ distilled water (2:5:1.5, v/v/v).
HPAEC-PAD was carried out using DX 500 Chromatography System (Dionex, Sunnyvale, CA, USA) and a CarboPac PA-1 column (4 × 250 mm; Dionex). Sizeexclusion HPLC was performed using UltiMateä 3000 RSLCnano System (Thermo Fisher Scientific, Inc., Waltham, MA, USA), with an OHpak SB-802.5 column (8.0 × 300 mm; Shodex, New York, NY, USA). The mobile column phase was distilled water, the flow rate was 0.4 mL/min, and the column oven temperature was 35°C. 1H-NMR spectroscopy was carried out with JEOL JNM-LA400 with LFG (JEOL, Tokyo, Japan) at 80°C.
Oligosaccharides were reacted with several carbohydrate hydrolases and reacted at 37°C for 1 h: 10 mU α-amylase (10 mU; Sigma-Aldrich), α-glucosidase (100 mU; Sigma-Aldrich), amyloglucosidase (520 mU; Sigma-Aldrich), β-glucosidase (100 mU; Sigma-Aldrich), and pullulanase M1 (1.4 U; Megazyme, Chicago, IL, USA).
To identify monosaccharides constituting oligosaccharides, 1 mL of 4 M trifluoroacetic acid (TFA) was added to 1 mL oligosaccharide solution (0.2%, w/v). After reacting at 121°C for 4 h, the solvent was completely removed with nitrogen gas. Oligosaccharides degraded by acid were analyzed by TLC and HPAEC-PAD.
The selected probiotic strains were 24 strains obtained from the Korean Culture Center of Microorganisms (KCCM; Seoul, Korea). Lactobacillus acidophilus KCCM 32820, Lactiplantibacillus pentosus KCCM 40997, Ligilactobacillus salivarius KCCM 40210, Lactobacillus johnsonii KCCM 41274, Lacticaseibacillus casei KCCM 12452, Limosilactobacillus fermentum KCCM 35469, Lactobacillus helveticus KCCM 40989, Lacticaseibacillus paracasei KCCM 40995, Lactiplantibacillus plantarum KCCM 12116, Lacticaseibacillus rhamnosus KCCM 32405, Enterococcus faecium KCCM 12118, Streptococcus thermophilus KCCM 35496, Lactococcus lactis KCCM 40104, Leuconostoc citreum KCCM 12030, Leuconostoc mesenteroides KCCM 11325, Pediococcus pentosaceus KCCM 11902, W. cibaria KCCM 41287, Bifidobacterium adolescentis KCCM 11206, Bifidobacterium longum KCCM 11953, Bifidobacterium animalis KCCM 11209, Bifidobacterium bifidum KCCM 12096, and Bifidobacterium breve KCCM 42255 were used. These strains were cultured in Lactobacilli de Man, Rogosa and Sharpe (MRS; BD) consisting of 2 g dipotassium hydrogen phosphate, 20 g glucose, 0.2 g magnesium sulfate heptahydrate, 0.05 g manganous sulfate tetrahydrate, 8 g meat extract, 10 g peptone, 5 g sodium acetate trihydrate, 2 g triammonium citrate, and 4 g yeast extract, per liter, at 37°C for 24 h.
Saccharomyces cerevisiae KCCM 50549 and Zygosaccharomyces rouxii KCCM 12066 were cultured in yeast malt (YM) medium (BD) consisting of 10 g glucose, 3 g malt extract, 5 g peptone, and 3 g yeast extract, per liter, at 30°C for 48 h.
To determine the prebiotic effect of oligosaccharides, modified MRS (m-MRS) was used as the LAB culture medium, and modified YM (m-YM) was used for the yeast culture, which had no carbon sources, as a negative control (Kim et al., 2021). Oligosaccharides were added to the medium at a concentration of 1% (w/v). m-MRS and m-YM, each containing 1% glucose, were used as positive controls. FOS (from chicory; Sigma-Aldrich) were also added to the growth medium at a concentration of 1% (w/v) as a reference.
The culture broth of probiotic strains was inoculated into the above-described media and sampled at 0, 6, 12, 24, and 48 h. LAB and bifidobacterial cells were spread onto an MRS agar plate, and yeast cells were spread onto a YM agar plate. The viable cell number was calculated after the plates were incubated at 37°C for 24 h.
Data are the mean±SD from triplicate experiments. Statistical analyses were performed using SPSS version 23 (SPSS, Inc., Chicago, IL, USA). Statistical significance between groups was determined by one-way analysis of variance, followed by Duncan’s multiple range test (p < 0.05).
Results and Discussion
To obtain LAB that produce oligosaccharides, LAB isolated from various sources were cultured on a PES agar plate. LAB with glycosyltransferase activity synthesize oligosaccharides or polysaccharides to form viscous colonies. Among 974 strains, 187 strains produced viscous colonies on the PES agar plate; the oligosaccharides they produced were analyzed using TLC, and their glycosyltransferase activities were also checked. Among 187 strains, strain YRK005, which had excellent oligosaccharide production ability, was finally selected for this study.
To identify the strain, its genomic DNA was isolated, and the phylogenetic tree was analyzed based on the nucleotide sequence of 16S rRNA. Strain YRK005 was identified as W. cibaria, and SEM analysis showed that strain YRK005 were short rods of 1.3 to 1.7 0.6 to 0.7 μm in size, as shown in Fig. 1. W. cibaria YRK005 was Gram-positive, positive in the KOH test, and catalase-negative, consistent with the characteristics of LAB.

Weissella spp. are LAB mainly isolated from kimchi, and W. cibaria is LAB mainly isolated from kimchi and has been commercially available as probiotics (Lee et al., 2018b;Kang et al., 2020).
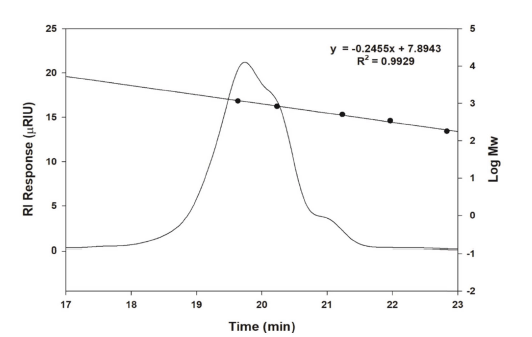
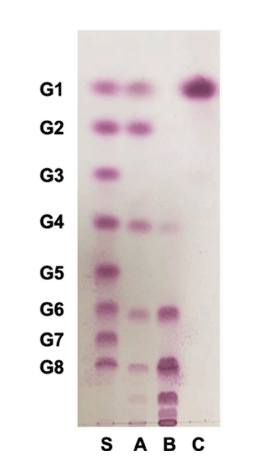
Among 974 LAB strains examined, W. cibaria YRK005 produced oligosaccharides in good yield using sucrose and maltose as donor and acceptor molecules, respectively, and the oligosaccharides produced from W. cibaria YRK005 were purified to determine their structure. Size-exclusion HPLC was used to obtain oligosaccharides produced from W. cibaria YRK005, and oligosaccharides with a degree of polymerization (DP) from 4 to 10 were obtained (Fig. 2, lane B).
Oligosaccharides are short-chain polysaccharides with DP from 2 to 10 and could be synthesized by the action of glycosyltransferases, such as glucansucrase and dextransucrase, from LAB, including Leuconostoc spp., Weissella spp., and Lactobacillus spp. (van Hijum et al., 2006;Patel & Goyal, 2011). W. cibaria YRK005 produced oligosaccharides using glucansucrase, and the optimum conditions for oligosaccharide production were determined using response surface methodology as described previously (Kim, 2021).
In a previous study, gluco-oligosaccharides with DP from 4 to 9 produced from Leuconostoc lactis CCK940 were purified, and their structure was determined (Lee et al., 2019).
The molecular weight of oligosaccharides from W. cibaria YRK005, measured by size-exclusion HPLC, was determined to be 1.12×103 (Fig. 3). Dextransucrase (EC 2.4.1.5) is a glycosyltransferase that catalyzes the transfer of an α- glucopyranosyl group from sucrose to form dextran, an α-l,6- linked polysaccharide with a molecular weight of >106 (Paul, Oriol et al., 1986). The molecular weights of chitosan and pectin oligosaccharides were confirmed to be 1000 to 1600 and 1000 to 3000 Da, respectively (Li, Xia et al., 2016). The molecular weight of oligosaccharides from W. cibaria YRK005 was less than those of other oligosaccharides reported. To date, studies on prebiotic oligosaccharides indicated that fermentation of oligosaccharides with low molecular weight by LAB was faster than those with higher molecular weight. This is because low molecular weight per unit mass has more nonreducing ends that are susceptible to be attacked by enzymes produced by Bifidobacterium (Gibson et al., 2004).
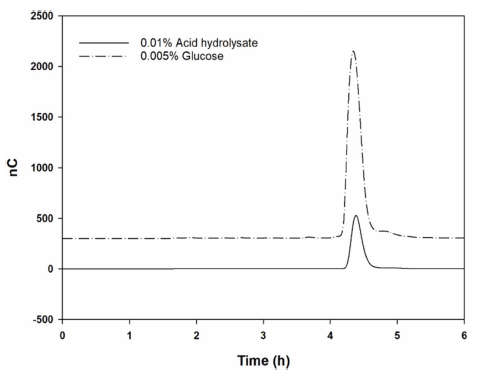
To determine the composition of monosaccharides constituting oligosaccharides from W. cibaria YRK005, the oligosaccharides were completely hydrolyzed with TFA, and the monosaccharide composition was analyzed by TLC and HPAEC-PAD. As shown in Fig. 2 (lane C) and 4, the hydrolyzed product was determined to be glucose only.
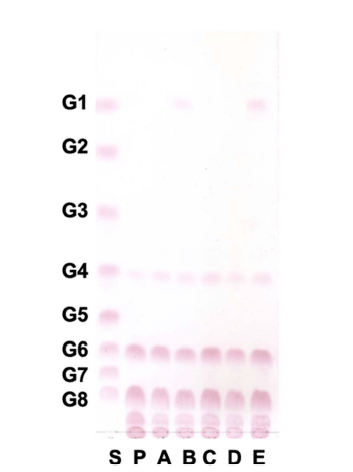
Oligosaccharides from W. cibaria YRK005 were hydrolyzed with several sugar hydrolases, and the glycosidic linkage was analyzed by TLC. As shown in Fig. 5, oligosaccharides from W. cibaria YRK005 were not hydrolyzed by α-amylase, α- glucosidase, and β-glucosidase but were hydrolyzed by amyloglucosidase and pullulanase M1. α-Amylase (EC 3.2.1.1) and α-glucosidase (EC 3.2.1.20) are typical carbohydrate hydrolases present in mammals and cleave α-1,4 glucosidic bonds (Tan et al., 2017). β-Glucosidase (EC 3.2.1.21) releases a glucose unit from the nonreducing end of the β-1,4 or β-1,3 glycosidic bonds of oligosaccharides. Amyloglucosidase (EC 3.2.1.3) cleaves the α-1,4 and α-1,6 bonds of oligosaccharides, and pullulanase M1 cleaves α-1,6 bonds. The results indicated that glucose units in oligosaccharides from W. cibaria YRK005 were linked mainly by α-1,6 linkage.
When glycosidic linkages of oligosaccharides from W. cibaria YRK005 were examined by 1H-NMR spectroscopy, the linkages in oligosaccharides from W. cibaria YRK005 were all α-linked. Oligosaccharides from W. cibaria YRK005 had 6.2% of α-1,4 linkages and 93.8% of α-1,6 linkages and a branching ratio of α-1,4 to α-1,6 linkages of 0.1 (Table 1). Therefore, oligosaccharides from W. cibaria YRK005 are gluco-oligosaccharides with almost α-1,6 glycosidic linkages. Isomalto-oligosaccharides consist of two to five glucose units, which are α-1,6 linkages (Chockchaisawasdee & Poosaran 2013). Oligosaccharides from W. cibaria YRK005 had almost α-1,6 glycosidic linkages and could be considered as nearly isomalto-oligosaccharides.

|
W. cibaria CMGDEX3 is dextran mainly with α-1,6 glycosidic linkages and only 3.4% of α-1,4 linkages (Ahmed et al., 2012). Dextran from Weissella strains generally has a lower degree of branching compared to dextran from L. mesenteroides (Bounaix et al., 2009). Currently, there is increasing interest in dextran-producing strains to produce prebiotic gluco-oligosaccharides, and Lactobacilli and Weissella spp. are known as dextran-producing LAB (Remaud-Simeon et al., 2000;Maina et al., 2008).
The prebiotic activity of oligosaccharides from W. cibaria YRK005 was evaluated using 24 probiotic strains. The prebiotic activity was checked by supplementing 1% (w/v) oligosaccharides from W. cibaria YRK005 in m-MRS or m- YM and compared to the medium supplemented with 1% (w/ w) glucose as a positive control and 1% (w/w) FOS as a reference. Among 24 strains tested, 4 strains, B. adolescentis, L. acidophilus, L. pentosus, and Lc. lactis, showed promoted growth by oligosaccharides from W. cibaria YRK005 compared to the positive control or reference (Fig. 6).
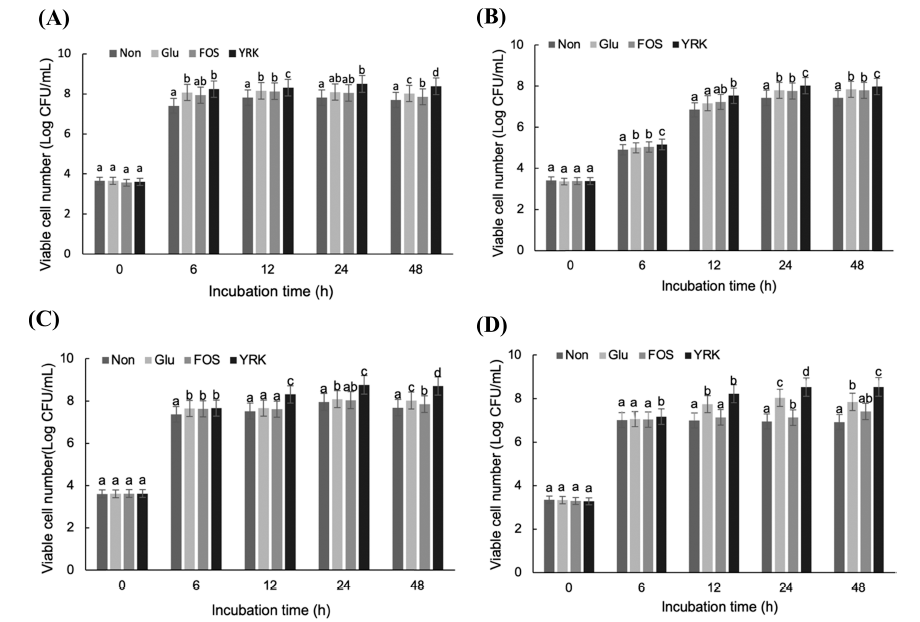
In m-MRS with oligosaccharides from W. cibaria YRK005, the viable cell number of B. adolescentis increased significantly at 24 and 48 h compared to other media (Fig. 6A). Bifidobacterium spp. carry multiple genes that catabolize carbohydrates and can grow using short-chain oligosaccharides. Bifidobacterium spp. can metabolize gluco-oligosaccharides more efficiently than Lactobacillus spp., Lactococcus spp., and Streptococcus spp. (Sarbini et al., 2014). In addition, the oligosaccharides with DP from 3 to 6 obtained from olive trees had prebiotic effects on B. adolescentis (Ruiz et al., 2017). L. acidophilus and Lc. lactis also showed promoted growth by the supplementation of oligosaccharides from W. cibaria YRK005 (Fig. 6B and D). L. pentosus also increased its growth significantly in m-MRS with oligosaccharides from W. cibaria YRK005 but did not use FOS for its growth (Fig. 6C). This result indicated that oligosaccharides from W. cibaria YRK005 had prebiotic activity on some probiotic strains.
Currently, although the prebiotic mechanism of oligosaccharides is not fully elucidated, their beneficial health effects are exerted by the composed monosaccharides, types of glycosidic linkages, and metabolic pathways for their consumption by probiotic strains, such as bifidobacteria and lactobacilli present in intestinal tracts of mammals (Bivolarski et al., 2018).
Recently, studies on gluco-oligosaccharide are focused on their prebiotic activity oligosaccharides; however, there are few reports on the prebiotic effects of gluco-oligosaccharides produced from W. cibaria strains (van Hijum et al., 2006;Hu et al., 2020). This study is the first to report the prebiotic potential of oligosaccharides produced from W. cibaria strains. Oligosaccharides from W. cibaria YRK005 promoted the growth of B. adolescentis, L. acidophilus, L. pentosus, and Lc. lactis, which are the main probiotic strains. This showed that oligosaccharides from W. cibaria YRK005 could be used as a potential prebiotic material.