Introduction
Exposure of raw materials for foods to heavy metal ions such as copper, lead, nickel and cadmium can cause a lot of serious problems to plants, animals and humans like a growth inhibition and cancer (Dutton et al., 2011;Ali et al., 2019). Higher amounts of heavy metals are disposed from various industries such as mining, electroplating, leather tanning, pesticide and electrical appliance manufacturing (Oves et al., 2013;Al-Hamaidan et al., 2014). Especially, trophic transfer of heavy metals and metalloids in food chains indicates that the necessity for the removal of heavy metals in environment.
Among the heavy metal ions, the Cu (II) is essential metal to living beings for enzyme synthesis, tissues development and connective tissue, however, unregulated excessive level of the Cu (II) ingestion can lead to adverse effect (Bilal et al., 2013;Hu et al., 2015). Various physico-chemical treatment methods for removal of the Cu (II) include ion exchange, chemical precipitation and electrochemical treatment (Yargıç et al., 2015). However, these physico-chemical technologies have limitation such as energy intensive, high cost and secondary pollution products.
In recent years, biosorption of the Cu (II) using biosorbents has been studied by many researchers (Cid et al., 2015;Martín-Lara et al., 2016;Choińska-Pulit et al., 2018). In previous studies, the Cu (II) biosorption have been reported with various microorganisms such as Lysinibacillus fusiformis, Micrococcus luteus and Pseudomonas veronii (Vullo et al., 2008;Puyen et al., 2012;Mathivanan et al., 2016). The biosorption of microorganisms (bacteria-based bioremediation) was considered as a promising, eco-friendly and costeffective method (Govarthanan et al., 2016). Especially, the use of certified microorganisms like a “Generally Recognized as Safe (GRAS) or microbial food ingredients” is one of the acceptables due to their safety. For example, the Bacillus amyloliquefaciens strain usually isolated from foods and it was approved as a general food ingredient by Ministry of Food and Drug Safety (MFDS, South Korea). Furthermore, this strain has been used for decades to produce a variety fermented foods, including doenjang, kimchi and cheese (Song et al., 2018).
In this study, we isolated a Bacillus sp. SRCM 112835 from doenjang. This strain showed a high Cu (II) resistance and biosorption properties in aqueous solution. Batch experiments were carried out to determine biosorption efficiency and removal mechanism of the Cu (II) by Bacillus sp. SRCM 112835. The Bacillus sp. SRCM 112835 biomass characterization was carried out by the FT-IR and point of zero charge (pHpzc) analytical methods. We also determined the influence of the initial Cu (II) concentration, pH, biomass dosage and contact time for the optimized the environmental factors affecting.
Materials and Methods
The Cu (II) biosorption bacterial strain SRCM 112835 was isolated from Doenjang (Korean Fermented Soy Paste) which was purchased in local market (Sunchang, Korea). The Cu (II) solutions were prepared using CuSO4·5H2O (Analytical grade). The PEI (Polyethylenimine) branched was purchased from Sigma-Aldrich (St. Louis, MO, USA). Identification of SRCM 112835 strain was performed by Macrogen (Macrogen Inc., Seoul, Korea) using a universal primers 785F (5'- GGATTAGATACCCTGGTA-3') and 907R (5'-CCGTCAA TTCMTTTRAGTTT-3'). The 16S rRNA sequence results for species identification were analyzed using the database of EZBioCloud (https://www.ezbiocloud.net).
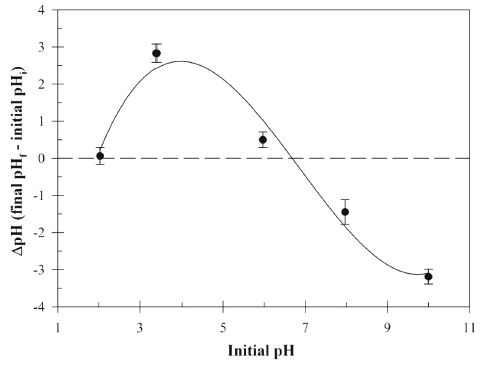
The Cu (II) concentration in aqueous solution was estimated using the UV-Vis spectrophotometer (Tecan Spark 10M, Tecan Trading AG, Maennedorp, Switzerland) at 630 nm using the polyethylenimine, a cationic polymer which has been used for detection of the Cu (II) by formation of cuprammonium complex (Wen et al., 2017). Fourier transform infrared (FT-IR) spectroscopy analysis was performed using Perkin-Elmer Spectrum 400 model FT-IR spectrometer (PerkinElmer, Inc., Shelton, CT, USA) to determine functional groups. The FR-IR spectra were collected in the 450-4,000 cm−1 wavenumber range. The pHpzc (point of zero charge) analysis was investigated using the Brouers and Al-Musawi method (Brouers & Al- Musawi, 2015). The initial pH values are adjusted using the 0.1M NaOH and 1.0 N HCl solution. The pHpzc (ΔpH = 0) of Bacillus sp. SRCM 112835 was estimated with 0.1 M NaCl solution. The pHpzc valuses are calculated by drawing a plot ΔpH (final pHf - initial pHi).
To determine biosorption properties of the Cu (II) by Bacillus sp. SRCM 112835, cell pellets were prepared from 24 h culture solution (Luria-Bertani broth) by centrifuging at 9168 xg. The precipitated cell pellets were washed several times with 0.1 M phosphate buffer solution (PBS, pH 7.0) and freeze-dried (-80°C, 5.0 Torr) for 48 h.
Biosorption experiments were performed to investigate the influence of pH (2.08-9.98), biomass dosage level (0.005-0.07 g/ 30mL), initial concentration (5.0-148.7mg/L) and contact time (0-180 min), respectively. The various pH (2.08, 3.81, 5.93, 8.06 and 9.98) solutions were titrated using the 0.1M NaOH and 1.0 N HCl solution. The influence of initial pH on the Cu (II) biosorption was investigated by 0.005 g of the biomass with a 30 mg/L of the Cu (II) solution. The chemical speciations of the CuSO4 (10 mg/L, 25°C) at different pH levels were calculated using Visual MINTEQ 3.1 (KTH Royal Inst., Stockholm, Sweden) software (Gustafsson, 2011). The Cu (II) adsorption capacity and removal rate (%) were calculated according as Eqs. (1) and (2) :
where qe (mg/g) is the adsorption capacity of the Cu (II); V (L) is the volume; C0 and Ct (mg/L) are initial and final Cu (II) concentration, and W (g) is the biomass weight of Bacillus sp. SRCM 112835.
The biosorption isotherms were analyzed using the Langmuir and Freundlich isotherm models (equilibrium model). To describe the adsorption mechanism, Langmuir and Freundlich isotherm models are widely applied (Limcharoensuk et al., 2015). The biosorption of the Cu (II) was carried out with 0.005 g of the biomass in initial 5.0-148.7 mg/L concentration (25°C) for 6 h. The equilibrium biosorption of the Cu (II) experimental data was fitted to the Langmuir and Freundlich isotherm models. The Langmuir and Freundlich isotherm model (non-linear form) equations are describe as Eqs. (3) and (4), respectively (Freundlich, 1907;Langmuir, 1918):
where qe (mg/g) is the adsorption capacity of the Cu (II) at equilibrium; qm (mg/g) is the maximum biosorption capacity; KL (L/mg) is the Langmuir isotherm constant (magnitude of the slope); Ce (mg/L) is the Cu (II) concentration at equilibrium; Kf is the Freundlich biosorption coefficient, and 1/n is an empirical parameter (adsorption intensity). In addition, the dimensionless separation factor (RL) is described as (Eq. 5):
where C0 (mg/L) is the highest initial Cu (II) concentration in aqueous solution.
The pseudo-first-order and pseudo-second-order models were applied to predict the biosorption rate and mechanism. The kinetic experiments were carried out using 0.01 g of Bacillus sp. SRCM 112835 biomass in a different initial concentration (30.3 and 58.7 mg/L) of the Cu (II) solution (30 mL, 25°C). The nonlinear form of pseudo-first-order and pseudo-second-order models were calculated according as Eqs. (6) and (7), respectively:
where qt and qe (mg/g) are the amount of the Cu (II) absorbed at time t (min) and equilibrium; k1 (min−1) is the kinetic rate constant of the pseudo-first-order model; k2 (g/mg/min) is the rate parameter of pseudo-second-order model.
Results and Discussion
In present study, the SRCM 112835 strain was selected from isolate strains collection of deonjang sample based on the Cu (II) resistance, safety and biosorption capacity. The partial 16S rRNA sequence of SRCM 112835 was analyzed by the Macrogen Inc., and then sequence was bootstrapped using the BioEdit Sequence Alignment Editor 7.2.5 program. The 16S rRNA sequence showed that the SRCM 112835 strain related to Bacillus species (B. subtilis 99.67%, B. siamensis 99.5%, and B. velezensis 99.5%) which was labeled as Bacillus sp. SRCM 112835.
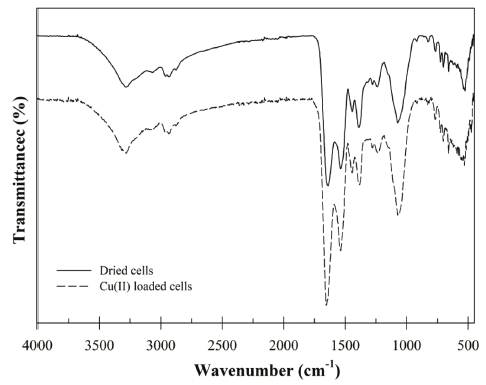
The functional groups of the surface of absorbent is one of the important factors to understand the biosorption mechanism. To identify functional group characteristics of the Bacillus sp. SRCM 112835, the FT-IR analysis was performed in the range of 450-4,000 cm−1 (Fig. 1). The FT-IR spectra shows the bands for the -OH stretching vibration (or N-H stretching of amine) at around 3,281 cm−1 (Yargıç et al., 2015). The peaks at 1648 and 1,537 cm−1 can be attributed from the amide I (amide C=O) and amide II (secondary amines) of protein (Bai et al., 2013). In addition, the spectra at the 2,932 and 1,445 cm−1 were assigned as the C-H and -COOH stretching. After biosorption of the Cu (II), the wavelength was shifted from 3,291 cm−1 to 3,281 cm−1 (-OH stretching vibration) and 1,445 cm−1 to 1,442 cm−1 (-COOH stretching vibration). These shift of peaks indicate that the -OH and -COOH groups are involved in biosorption of the Cu (II). Previously, similar results were reported for the hydroxyl and carboxyl group mainly involved in the Cu (II) sorption by the formation of surface complexes (Corapcioglu & Huang, 1987;Shim et al., 2015).
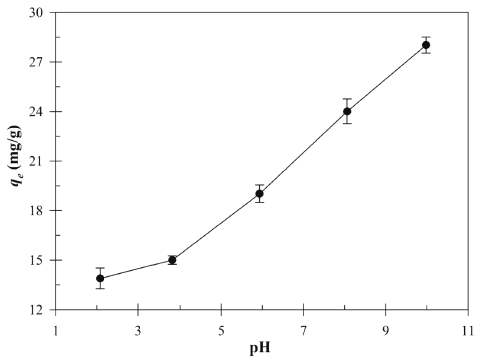
To determine the influence of initial pH on the Cu (II) biosorption, the biosorption experiments were carried out at various pH levels (2.08-9.98). The initial pH value has been recognized as one of the important factors for the biosorption process of the Cu (II) in aqueous solution due to degree of ionization and surface charge (Bueno et al., 2008). The Visual MINTEQ 3.1 was used to calculate the chemical speciations of 1.0M CuSO4 in different pH (2-10) ranges (Table 1). The MINTEQ calculation results suggested that Cu3(OH)42+ mainly dominate the Cu (II) speciation in the pH range 8-10.
Fig. 2 shows the effect of initial pH on the biosorption of the Cu (II) by Bacillus sp. SRCM 112835. The result indicates the increasing in the Cu (II) adsorption capacity with increasing initial pH. The biosorption capacity was peaked at an initial pH 9.98 showing 28.02 mg/g. Previously, a similar pH effect on the biosorption of the Cu (II) was reported (Bueno et al., 2008;Rodríguez-Tirado et al., 2012). As shown in Fig. 3, the pHpzc (ΔpH=0) of the Bacillus sp. SRCM 112835 was observed as pH 6.67. At a pH lower than pHpzc (below pH 6.67), Bacillus sp. SRCM 112835 was positively charged (protonated). Beyond the pHpzc, the surface of Bacillus sp. SRCM 112835 charged as negative and attracted positively charged ions.
The pH dependent behavior of the Cu (II) biosorption might be due to the interaction between the binding sites and Cu (II) ions. At lower pH, the binding sites of absorbent are protonated which results in a competition between the proton ions (H+) and Cu (II) cations for the binding sites of the Bacillus sp. SRCM 112835. However, as the pH increases, the functional groups of the binding site such as carboxyl, hydroxyl and amino groups are dissociated (Shi et al., 2017). Therefore, the greater biosorption of the Cu (II) observed at higher pH values.
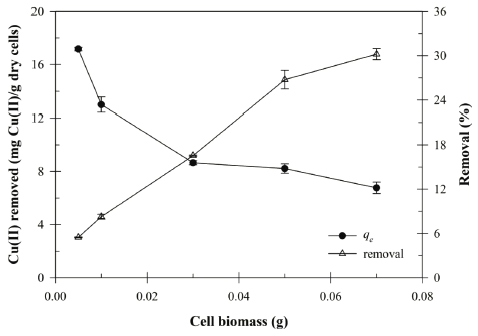
The biomass ranged from 0.005 to 0.07 g in 30 mL solution was used to determine the effect of Bacillus sp. SRCM 112835 biomass on the biosorption capacity and removal efficiency. As shown in Fig. 4, the removal efficiency of the Cu (II) increased with increasing biomass due to the increase of the biosorption surface area. The maximum biosorption capacity was reached to 30.19 mg/g at 0.07 g. However, the biosorption capacity (qe) of the Cu (II) gradually decreased with increasing cell dosage. The decreasing of biosorption capacity at high biomass dosage could be due to a partial cell aggregation (or screen effect). Previously, similar observation has been reported for biosorption of the Cu (II) in aqueous solution by Cladonia rangiformis hoffm and Rhodococcus opacus (Ekmekyapar et al., 2006;Bueno et al., 2008).
The biosorption capacity experimental data of the Cu (II) in different initial Cu (II) concentrations (5.0-148.7 mg/L) was plotted in Fig. 5. The non-linear form of Langmuir and Freundlich isotherm model parameters are given in Table 2. As shown in Table 2, the coefficients of determination (R2) of the Langmuir isotherm model (R2= 0.96) much higher than Freundlich isotherm model (R2= 0.89). Higher R2 value indicated that the Langmuir model was much better to describe the equilibrium biosorption of the Cu (II) by Bacillus sp. SRCM 112835 than Freundlich isotherm model. The Langmuir isotherm model is based on the assumption that the monolayer adsorption of absorbate on the surface of absorbent. Furthermore, the dimensionless constant separation factor (RL) was calculated to 0.05 (0 < RL < 1) which indicated the favorable biosorption condition (Weber & Chakravorti, 1974). The maximum biosorption capacity (qmax) was calculated to be 23.02mg/g.
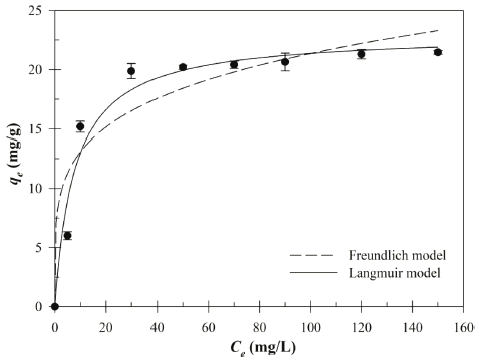
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | RL | KF | 1/n | R2 |
| 23.02 | 0.129 | 0.96 | 0.05 | 7.99 | 0.214 | 0.89 |
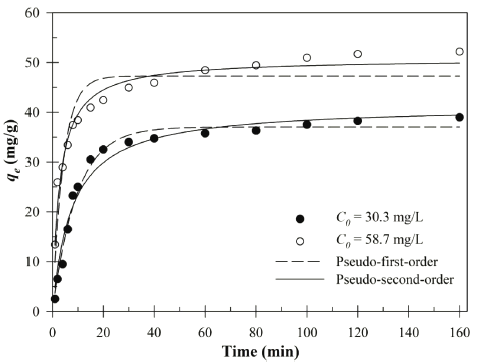
The kinetics of the Cu (II) by Bacillus sp. SRCM 112835 are shown in Fig. 6. In addition, the constants of kinetic models are shown in Table 3. The kinetic experimental data were plotted using pseudo-first-order and pseudo-second-order kinetic models (non-linear regression), respectively. The biosorption equilibrium of the Cu (II) was reached within 1 h in aqueous solution. The fast biosorption of the Cu (II) indicated the abundant active binding sites of Bacillus sp. SRCM 112835. The pseudo-second-order kinetic model described the best fit for the Cu (II) biosorption experimental data than pseudofirst- order kinetic model for both initial concentrations (30.3 and 58.7mg/L). This results suggest that a chemisorption was more favorable the biosorption of the Cu (II) by the Bacillus sp. SRCM 112835. The biosorption capacity values were calculated using the pseudo-second-order formula as 41.31 and 50.73 mg/g, respectively.
In this study, the SRCM 112835 strain was isolated from doenjang and identified as Bacillus sp. The maximum biosorption capacity of the Cu (II) was observed at higher initial pH levels and cell dosages. Furthermore, the biosorption isotherm and kinetic results showed that the Langmuir and pseudo-secondorder models well fitted. These results suggested the potential of the Bacillus sp. SRCM 112835 strain as a biosorbent for the Cu (II) in aqueous solution.