Introduction
Many studies have been done to predict and to quantify the changes in foods during processing and storage. However, fundamental information related to physiochemical changes and transport phenomena occurring in foods is still not enough. Magnetic resonance imaging (MRI) is a technology that has a great advantage for analyzing intact target samples nondestructively. Technology based on NMR provides key information without altering the phenomena happened in various food processing (McCarthy, 1994). Minghim et al. (2002) studied three-dimensional (3D) reconstruction using two-dimensional (2D) MR images of tropical fruits to characterize internal structure. Kim et al. (2008) utilized fast MRI technique to identify seeds and freeze damaged tissues of mandarin oranges. Patel et al. (2013) reviewed various developments in applications of MRI techniques for agricultural and food products. They concluded that MRI is a powerful tool to investigate biosystems non-invasively and non-destructively.
Much time and attention have been spent on investigating grains and beans using MRI techniques by many researchers. Heil et al. (1992) utilized MRI technique for modeling of water up-take process into dry bean. Duce and Hall (1995) examined hydration of a butter bean for 20 h using MRI technique. They removed the bean, out of water, imaged the bean and then re-immersed for the experiment. Song et al. (1998) demonstrated 3D magnetic resonance imaging capabilities to investigate the moisture distribution of a mature soft wheat grain during grain storage. Frank (2001) showed water distribution of coffee bean is heterogeneous using an MRI technique. Pietrzak et al. (2002) investigated water uptake and distribution analysis in a single soybean seed successfully using a high-resolution MRI system during hydration process for 22 h. However, water was removed from the sample tube during magnetic resonance (MR) imaging. Ishida et al. (2004) examined the loss of moisture from rice seeds and Ghosh et al. (2006) determined the moisture removal patterns in wheat during drying process. NMR and MRI techniques were used to monitor the water uptake of navy beans during cooking process (Zhang & McCarthy, 2013). They periodically took out and blotted dry the beans for MRI measurement. Many works showed that applications of MRI to grains and beans are helpful to understand physiochemical changes during the processing. However, all samples were removed from the water to acquire MR images due to disturbance from strong signal from water.
Water in coffee beans is very essential component both in physiological and chemical processes. Water penetration into coffee bean still needs more works on its movement or amount during hydration process. Therefore, MRI technique is perfect tool to characterize internal structure changes of intact coffee beans during hydration process nondestructively. The objective of this study is to investigate the changes of water distribution and physical dimensions of intact dried green coffee beans during whole hydration process using an MRI technique noninvasively and nondestructively.
Materials and Methods
Commercial dried ‘Robusta’ green coffee beans were purchased at a local store and used in this study.
Coffee beans were immersed in a glass bottle with deionized (DI) water and sealed up with paraffin film to control the evaporation of water from the bottle. Fig. 1 shows coffee bean layout prepared in a glass bottle with a diameter of 25 mm and length of 60 mm, and it was placed in a 35 mm diameter RF coil for MR measurement. Physical changes including weight, length, thickness, and width of coffee beans before and after hydration process were measured with an electronic balance (CJ-820E, ViBRA SHINKO DENSHI CO., LTD., Tokyo, Japan) and a vernier caliper.
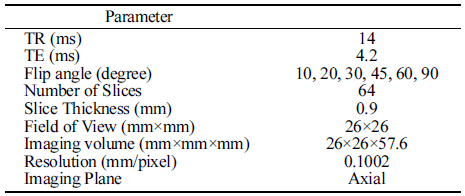
An industrial grade MRI system (M10, ASPECT Magnetic Technologies Ltd., Gesher, Israel) was used to acquire MR image data sets. The MRI system consists of 1 T (42 MHz) permanent horizontal bore magnet with 110 mm diameter. A conventional imaging pulse sequence named ‘Gradient Echo (GRE)’ with ‘axial’ image plane direction was used for MRI data acquisition. Table 1 shows MR imaging parameters for the imaging pulse sequence briefly used in the study. Repetition time (TR) of 14 ms and echo time (TE) of 4.2 ms were used to acquire clear MR images. For each test, a series of 64 images data set with 256×256 pixels were acquired as shown in Fig. 2. The slice thickness was 0.9 mm and the field of view (FOV) of the acquired image was 26×26 mm and. So, we can obtain 3D volume data with a spatial information of (26×26×0.9)×64 mm3. The hydration process of dried coffee beans lasted for 45 h without any intentional disturbance. Image data sets were acquired in every 10 min up to 6 h to examine water movement in detail, and then three more data sets were acquired at 9 h, 12 h and 24 h after hydration process has started.
A commercial image processing software (MATLAB R2017b with Image Processing Toolbox, Mathworks Inc., Natick, MA, USA) was used to process ‘Digital Imaging and Communications in Medicine’ (DICOM) images acquired. A customized software was developed to analyze a series of 64 MR images shown in Fig. 3. A center slice image from the series of image data sets at same position was used for further processing. Before processing each selected image, we extracted the oil reference region, and then normalized all the images to compare the data in the same condition if the moisture content is increased. Ten coffee beans were extracted from the region of interest and compared each mean value from the images.
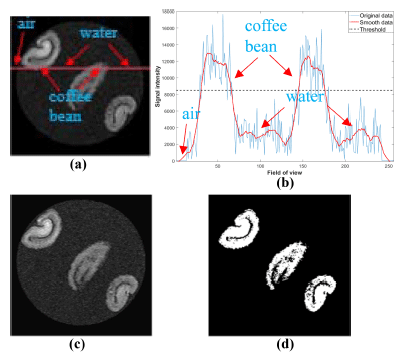
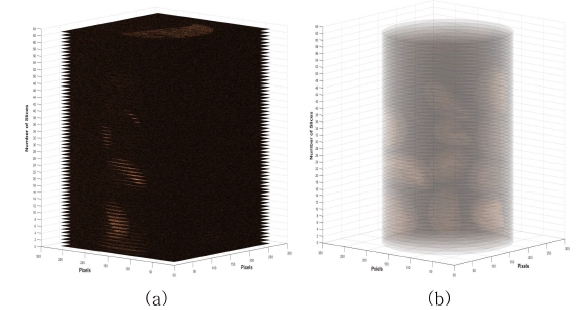
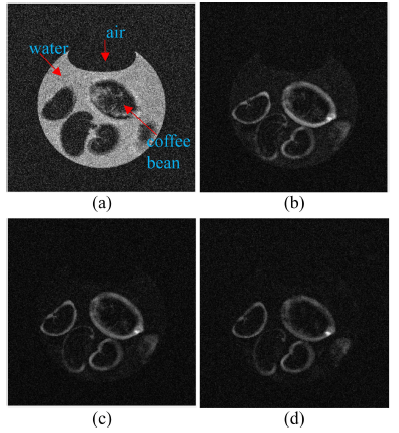
Acquired MR images are DICOM file type with a 16-bit integer value. To check the amount of water penetration into the coffee beans in detail, it needs to extract region of interest (ROI) of target coffee bean. Fig. 4 shows image processing steps for isolating target coffee beans. Fig. 4(a) shows an original MR image acquired during the hydration process. We can observe strong signals from the coffee beans and very few signals from background noise as shown in Fig. 4(b). From one-dimensional (1D) profile image data analysis, simple lowpass filter technique is enough to get noise removed images. To remove background noises, we applied lowpass filter to the original image and obtained noise removed image as shown in Fig. 4(c). Moreover, suitable threshold value is needed to get binary image of target beans from 1D profile image data analysis as shown in Fig. 4(d). We can successfully isolate each beans in the acquired MR images using the binary images. Fig. 5 shows an example of 3D reconstructed MR images using acquired 64 images before and after applying an image processing technique to original MR images.
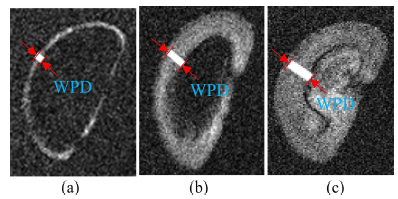
To measure the water penetration depth of the sample from the MR images, the measurement was done on the MR images as shown in Fig. 6.
Results and Discussion
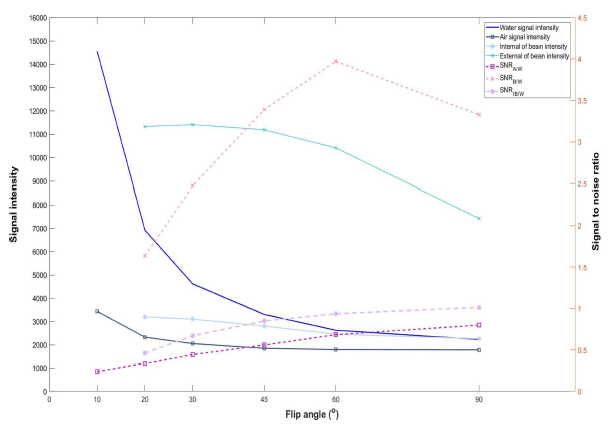
Generally, a pixel intensity of an MR image depends on the amount of water content in a target volume. Therefore, MR imaging of beans only in water is very difficult due to strong signals generated from water. To acquire signals from water penetrated into the bean a special technique is need to suppress strong signals from water surrounding the beans. In this study, we found change of a flip angle, one of typical GRE pulse sequence imaging parameters, generates different image intensity at the same part. Fig. 7 shows intensity variations of GRE images acquired with different flip angles. When the flip angle is 10° , water part generates stronger signal than coffee beans and air parts. However, when we increase the flip angle, the signal from the water part is getting weaker than outer layer of coffee beans. In this study, we tried to find optimal flip angle generating strong signal from water penetrated regions of coffee beans and weak signal from water unpenetrated regions of coffee beans, water surrounds coffee beans, and air regions. We analyzed MR images acquired with six different flip angles of 10° , 20° , 30° , 45° , 60° , and 90° . Fig. 8 shows signal intensity and SNR changes with different flip angles. The signal intensity from air and coffee bean parts decreases gradually with the increase of flip angle. However, the signal intensity from water parts decreases drastically with the increase of flip angle. Therefore, we analyzed variations of signal-to-noise ratio (SNR) of air (SNRA/W), water penetrated region (SNRB/W), water unpenetrated internal region (SNRIB/W) of coffee beans based on signal of water region. SNRA/W and SNRIB/W are gradually increase with the increase of flip angle. However, SNRB/W is increases up to flip angle of 60° but decreases after that value. Therefore, to investigate hydration process without disturbance an optimal flip angle is 60° .
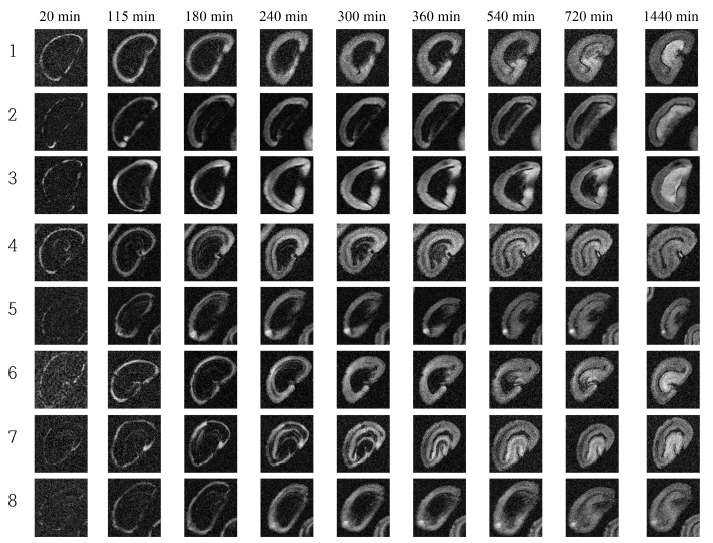
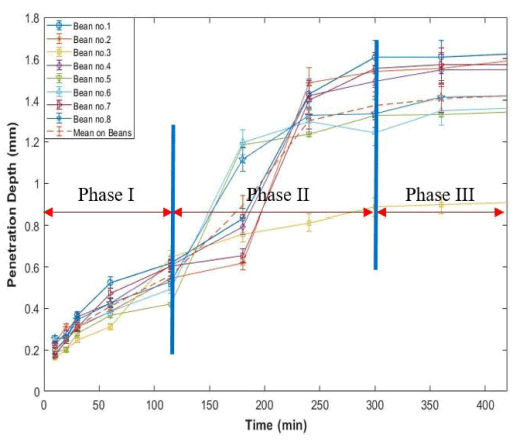
High-resolution MR images representing hydration process of dried green coffee beans were acquired with a developed MRI pulse sequence. Eight out of 11 coffee beans were analyzed intensively. Fig. 9 shows MR images of selected coffee beans with different water soaking times. Water penetrates into the bean as water soaking time increases shown in Fig. 9. Generally, water penetrated region generates strong signal and the region is increasing as time increases. Usually, water penetrates into outer layer of coffee bean about 20 min after the process. Fig. 10 shows the changes of water penetration depth of each coffee bean during hydration process. The hydration process of coffee bean can be divided into theree phases. At phase I (first 2 h of the process), water enters into beans at a relatively moderate rate. At phase II (between 2 h and 5 h of the process), significant water penetration depth change was observed and the process developed at a relatively rapid rate. At phase III (after 5 h of the process), the process is in steady state. Usually, no more rapid water movement phenomena were observed during this phase. Similar process pattern was observed but different water movement mechanism was mentioned in water movement during soybean hydration process (Pietrzak et al., 2002). The physiochemical properties of various beans play an important role in the movement of water inside seeds during hydration process.
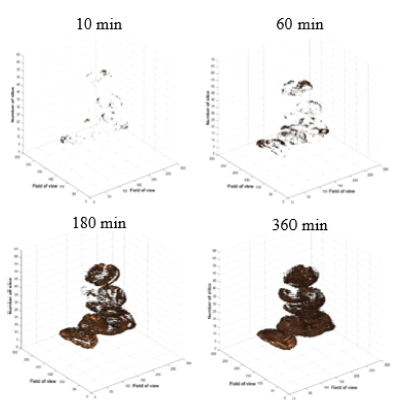
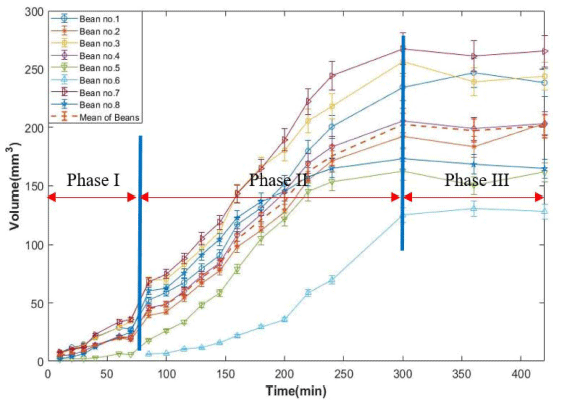
The changes of penetrated water volume into the bean was measured as calculating the sum of pixels from each coffee bean acquired different hydration times. Fig. 11 shows visualization of 3D reconstructed MR images during hydration process. In this figure, the water starts to penetrate into beans after 10 min of the process. Fig. 12 shows water volume changes of each coffee bean during the process. The pattern of the process is similar to the analysis of water penetration depth change (Fig. 10). However, Phase II starts early about 45 min compared to the penetration depth analysis in this process. This indicates that water moves into the dried bean and starts to fill up hollow space between tissues in outer part of the bean. Then, the water penetrates into the inner part of the bean. The volume change process nearly ends at after 5 h of soaking like water penetration depth analysis. Usually, volume of each bean increases linearly up to 5 h after hydration process has started. Volume change of each bean indicates the change of weight of each bean during hydration process. Similar results were obtained in weight changes during water uptaking process of green coffee beans (Shin, 2019).
Changes of weight, length, width, and thickness of 11 coffee beans were measured before and after the process using a balance and a vernier caliper. Table 2 shows mean values and standard deviations of physical factors of coffee beans before and after the process. The weight, length, width, and thickness of coffee beans increase about 85.4%, 14.9%, 18.2%, and 26.7% respectively. The changes of length and width estimated from image analysis were 17.3% and 19.8% respectively. This MRI experiment was done without disturbing the samples during the hydration process. Therefore, the real weight changes were not measured. This result gives us more challenge for utilizing MRI in measuring dimension changes occurred during food processing.

|
Summary
In this study, MRI technique was utilized with a helping of image processing techniques to investigate the hydration process of intact dried green coffee beans. An optimal GRE pulse sequence was developed to investigate the hydration process without disturbing the system. A region containing coffee beans only was extracted from the MR images of target coffee beans. MR image analysis was done in 2D and 3D imaging spaces. The results of 2D analysis showed that we could visualize the changes of water penetration depth inside of intact coffee beans during whole hydration process. In addition, the results of 3D analysis showed that more clear understanding of hydration process of dried coffee beans. This study showed that the changes of water distribution and physical dimension of coffee beans during hydration process in 2D and 3D imaging spaces using MRI technique noninvasively and nondestructively. MRI may be used as a fast analyzing tool in various food engineering fields.