Introduction
According to a report by the World Health Organization, per capita alcohol consumption has been on the rise, increasing from 5.5 L in 2005 to 6.4 L in 2016. As of 2019, the annual alcohol consumption in Korea was 8.7 L, which was higher than that of Japan (7.1 L) and Italy (7.7 L). From a global perspective, South Korea is one of the countries with high annual alcohol consumption (WHO, 2018).
Alcohol metabolism is a complex process in humans. A fraction (approximately 2-8%) of alcohol is expelled through respiration, perspiration, and urination, and the remaining (approximately 92-98%) is predominantly absorbed in the small intestine and stomach, reaching peak absorption about 25 minutes after ingestion (Lee et al., 2015). Once absorbed in the body, alcohol is primarily metabolized in the cytoplasm by alcohol dehydrogenase to acetaldehyde. Acetaldehyde is metabolized to yield acetic acid, and ultimately the end product of aldehyde dehydrogenase within the mitochondria (Edenberg, 2007). Notably, acetaldehyde, a primary byproduct of alcohol metabolism causes several side effects such as flushing of the face, headaches, palpitations, hypotension, nausea, and vomiting, while exerting toxic effects on diverse physiological systems (Wang et al., 2016).
Hangovers occur as a consequence of excessive alcohol consumption. The condition manifests as physical or mental discomfort along with diminished work performance. Hangovers are frequently observed in regular alcohol consumers and cause physical unease and significant social and economic burdens (Wiese et al., 2000;Verster, 2008). Typical hangover symptoms include thirst, fatigue, and headaches which are accentuated by dehydration resulting from residual alcohol in the body, electrolyte imbalance, gastrointestinal disturbance, low blood sugar levels, and disruption in sleep and circadian rhythms (Penning et al., 2012). The severity of hangovers varies depending on several genetic and environmental factors (Verster et al., 2019).
In modern society, increasing income levels have changed the perspectives on quality of life, and people are living longer, hence, the focus on natural substances that promote wellbeing has increased. Similarly, a surge of interest has been observed in hangover remedies that utilize natural ingredients with minimal side effects (Hwang et al., 2019;Je et al., 2021;Kim et al., 2021;Choi et al., 2023). Animal studies have reported that the natural fermented extracts of rice germ and soybean can reduce blood alcohol and acetaldehyde levels by modulating alcohol-metabolizing enzymes (Lee et al., 2009).
Therefore, we conducted a crossover trial to evaluate the efficacy and safety of DA-5521, which contains fermented rice germ and soybean extract, in alleviating hangover symptoms in individuals accustomed to experiencing hangovers following alcohol consumption, comparing it with a placebo.
Materials and Methods
This study aimed to assess the safety and efficacy of DA-5521 in alleviating hangover symptoms in individuals aged 19-50 years who had experienced a hangover following alcohol consumption, in comparison to a placebo. This study was approved by the institutional review board of Jeonbuk National University Hospital (approval no. CUH 2020-07-044-001) and registered with the Clinical Research Information Service of the Republic of Korea (registration number: KCT0008927). This study was conducted in accordance with the guidelines of the Declaration of Helsinki and complied with the Korean Good Clinical Practice guidelines.
We recruited 32 individuals who met the inclusion and exclusion criteria for human test participants and designed a double-blind, randomized, crossover trial with a placebo control design. To ensure the inclusion and exclusion criteria, we comprehensively assessed demographic surveys, physical examinations, clinical pathology examinations, medical and medication history surveys, physical measurements (height and weight), vital sign (blood pressure and pulse) monitoring, electrocardiogram examinations, and used a questionnaire to assess drinking habits. Inclusion criteria were as follows: 1) Adult males aged 19 to 50 years old; 2) Individuals who have experienced hangovers after drinking; 3) Those who agreed to participate in the clinical trial and signed the Informed Consent Form. Exclusion criteria included: 1) Individuals unable to consume more than 100 mL of hard liquor; 2) Those undergoing treatment for severe conditions including cardiovascular, immune, respiratory, hepatobiliary (liver and gallbladder), renal and urinary, neurological, musculoskeletal, mental, infectious diseases, or malignant tumors; 3) Individuals with digestive ulcers or reflux esophagitis, a history of significant gastrointestinal diseases (e.g., Crohn’s disease) affecting test food absorption, or major gastrointestinal surgeries, except simple appendectomy or hernia surgery; 4) Individuals with alcohol use disorder or recent significant alcohol consumption that may interfere with the test; 5) Those continuously taking medication believed to affect alcohol metabolism (e.g., Antabuse-class drugs) or drugs with a risk of gastrointestinal bleeding (e.g., warfarin, clopidogrel, aspirin, nonsteroidal anti-inflammatory drugs); 6) Those who have taken medications or health-functional foods related to liver function improvement or drugs inducing or inhibiting drug metabolism enzymes within the specified time frames before the screening visit; 7) Individuals with significantly abnormal levels of aspartate aminotransferase (glutamic oxaloacetic transaminase), alanine aminotransferase (glutamic pyruvic transaminase), creatinine, thyroid stimulating hormone, fasting blood sugar, or uncontrolled hypertension; 8) Those who have participated in another interventional clinical trial within the past 3 months or plan to participate in another during this clinical trial; 9) Individuals sensitive or allergic to the ingredients of the test food; 10) Those deemed inappropriate for the trial by the principal investigator. After comprehensive screening tests, a total of 24 participants met the inclusion and exclusion criteria and were enrolled within four weeks following the screening. All participants provided informed consent prior to the trial.
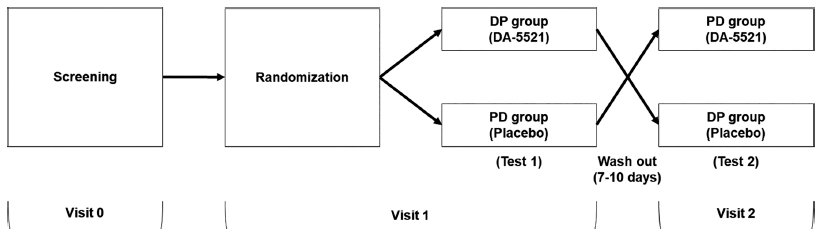
Individuals were allocated to either the DP group (visit two: DA-5521, visit three: placebo) or the PD group (visit two: placebo, visit three: DA-5521) and consumed either the DA-5521 or a placebo by a double-blind protocol. During visit two, 12 individuals from the DP group were administered DA-5521, whereas 12 individuals from the PD group received the placebo. Subsequently, during visit three, one participant was excluded from each group, resulting in 11 individuals from the DP group receiving the placebo and 11 individuals from the PD group receiving DA-5521 (Fig. 1). Following visit two, an intermission period of 7-10 days preceded visit three. Individuals were excluded if the attending physician determined that the test could not proceed during the trial period, or if they declined to participate (Fig. 2). Individuals were instructed to abstain from alcohol for 48 h before visits two and three. During visits two and three, the participants ingested DA-5521 for 10 min (±1 min), followed by the consumption of alcohol (100 mL of liquor, alcohol 20%) and 100 mL of water, each drink consumed at 2 min intervals. Furthermore, 4 h after drinking, the participants were provided with the standard meal and water (250 mL).
The individuals participating in the study arrived at the hospital after fasting for 8 h before their first visit. During the visit, blood samples were collected, and the following parameters were assessed: clinical pathology assessments for visits two and three were conducted concurrently with the final blood sample collection, 10 h (±5 min) after alcohol consumption. Similarly, a urine test was performed concurrently with the final blood collection, 10 h (±30 min) after alcohol consumption. The safety of the intervention was evaluated by assessing the frequency and severity of adverse reactions and abnormal findings on hematological and blood chemical tests, urine tests, vital signs (blood pressure and pulse), and physical measurements (weight). In case of any clinically significant findings among the abnormal values of clinical pathology tests, vital signs, or electrocardiogram results, these were recorded in the adverse reaction record table for each subject in the case report form (CRF) and evaluated through statistical analysis.
DA-5521 comprises a combination of fermented rice germ and soybean extract (RSE-α), vitamin B complex powder, kudzu root extract powder, taurine, green tea catechin extract, willow bark dry extract, maltol, glycine, AWACUT CP (dextrin, maltose, glycine ester of fatty acids, lecithin), Scutellaria baicalensis root extract, processed oligosaccharide products, erythritol, steviol glycosides, drink flavor, citric acid, and trisodium citrate. Among the above ingredients, the main ingredient is RSE-α and its content is 0.4 g/100 mL. The placebo was prepared using distilled water, designed to replicate the taste and color profiles of DA-5521. The participants were instructed to ingest 100 mL of either DA-5521 or the placebo before alcohol consumption.
To confirm the hangover-relieving effect of DA-5521, a biochemical analysis was performed before and after the participants consumed DA-5521. During visits two and three, the participants’ blood/breath alcohol and blood acetaldehyde concentrations were measured before ingestion of DA-5521 (-5 min) and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 10 h (±5 min) after the drink. The breath alcohol concentration was determined by quantifying the alcohol content in the exhaled breath. Blood samples were collected in anticoagulant tubes containing potassium ethylenediaminetetraacetic acid (BD Biosciences, San Jose, CA, USA) at baseline and 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 10 h after alcohol administration. Blood alcohol concentration was determined using headspace gas chromatography with flame ionization detection (HS-GC-FID) as described by Kristoffersen et al. (2006). For each sample, 100 μL of whole blood was diluted with 1000 μL of an internal standard solution in vials prepared for the HS-GC-FID system (Model 6890GC-FID, Agilent Technologies, Santa Clara, CA, USA), equipped with a headspace autosampler (Model G1888A, Agilent Technologies, Santa Clara, CA, USA). Analytical conditions were set as follows: using a DB-624 column (30 m × 0.251 mm × 1.40 μm; Agilent Technologies, Wilmington, DE, USA), the oven temperature program was set at 40°C with a 3-minute hold, followed by a ramp of 10°C/min up to 260°C, with a final 5-minute hold. The headspace oven temperature was maintained at 80°C with a sample heating time of 15 min. Blood acetaldehyde concentrations were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with multiple reaction monitoring (MRM), according to the method described by Ochs et al. (2015). Briefly, 1000 μL of whole blood was added to each vial containing a mixture of 1 mL of saturated sodium nitrite solution and 100 μL of acetone as the internal standard. After the addition of a 2, 4-dinitrophenylhydrazine (DNPH) cartridge, the reaction was allowed to proceed for 24 h in the dark. The analytes were extracted using 1 mL of acetonitrile and analyzed using a 6410 Triple Quad LC-MS/MS system (Agilent Technologies, Wilmington, NC, USA). The analytical column used was a reverse-phase Shiseido CAPCELL C18 column (5 μm, 2.0 mm × 10 cm). The flow rate was set to 0.23 mL/min, utilizing a gradient elution of water and acetonitrile containing 0.1% formic acid. The fragmentation and collision voltages were set to 100 V and 10 V, respectively. Ion detection was performed in MRM mode, monitoring the transition pairs of m/z 225.1 to 208.3 (aldehyde-DNPH). Data acquisition was performed using MassHunter Software (Version B.04.00). An electrochemical alcohol tester (Creative Applications of Sensors, Korea) was used to measure the breath alcohol levels.
Blood alcohol and acetaldehyde concentrations were assessed before the ingestion of DA-5521 (-5 min) and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, and 10 h (±5 min) following alcohol consumption. A graph illustrating the rate of absorption within the body was generated using the aforementioned data. Utilizing the generated graph, we computed and analyzed the maximum blood concentration (Cmax), time to reach maximum blood concentration (Tmax), and the area under the concentration-time curve (AUC, Area Under the Curve). These metrics represented the rates of bioabsorption over the entire duration of blood alcohol, blood acetaldehyde, and breath alcohol consumption.
Statistical analyses were performed using SAS (Version 9.4, SAS Institute, Cary, North Carolina, USA). A two-tailed test was used at a significance level of 0.05. The results for the measurements were presented as mean±standard deviation, and a significant difference between groups was presented at p-value<0.05.
Results and Discussion
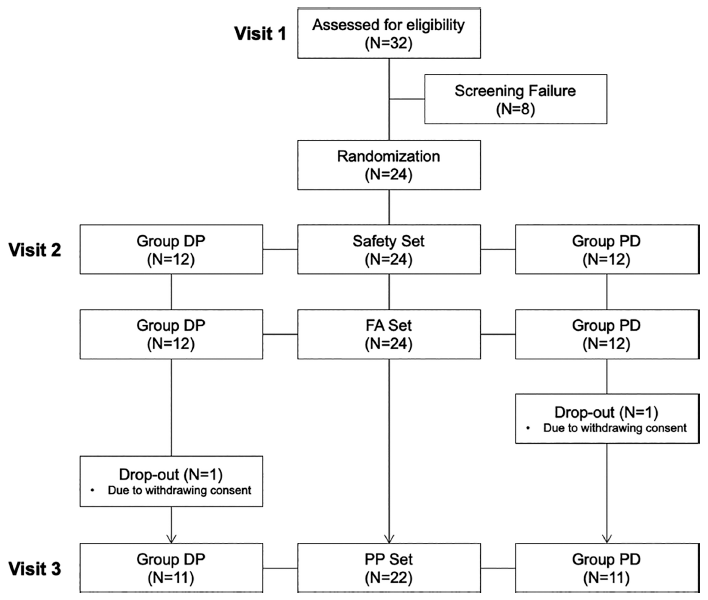
In this study, a cohort of 32 individuals were screened for eligibility. A total of 24 individuals (12 each in the DP group and the PD group) met the eligibility criteria and underwent randomization. The study procedures were exclusively carried out on the individuals who completed the full trial without any significant violations that could have impacted the results. One individual in the PD group withdrew consent and discontinued participation. Additionally, among the participants whose breath alcohol concentrations exceeded 0.1% after 0.5 h of drinking during visit two, one participant exhibited a test value in visit two that was twice as high as that in visit three. Consequently, 22 participants (11 each in the DP group and 11 in the PD group) were included in the analysis, following the exclusion of participants due to deviations from the crossover design (Fig. 2).
The demographic characteristics of the participants are displayed in Table 1. Demographic information of all participants was incorporated and group comparisons were conducted before the trial to identify various factors, including smoking and exercise habits (Verster et al., 2010). The DP group had an average age of 24.09±1.04 years, which was not statistically significantly different from the PD group which had an average age of 24.55±0.69 years, (p = 0.3090). Further, no statistically significant differences in occupation, exercise status, or average alcohol consumption were observed between the DP and PD groups, implying a degree of comparability between the groups.
A hangover is experienced after alcohol consumption, during which the alcohol absorbed from the stomach and small intestine is transported through the bloodstream to the liver, where it undergoes metabolism by the enzyme alcohol dehydrogenase to acetaldehyde. The acetaldehyde generated during this process is highly volatile and diffusible and is recognized as a toxic compound responsible for the majority of hangover-related symptoms (Lieber, 1992). Consequently, excessive alcohol consumption leads to the accumulation of highly toxic acetaldehyde in the body, which circulates in the bloodstream to induce hangover symptoms and contributes to liver cell damage (Hawkins and Kalant, 1972;Lieber, 1992). This suggests that improvements in blood alcohol and acetaldehyde levels can be important in evaluating the effectiveness of improving hangovers.
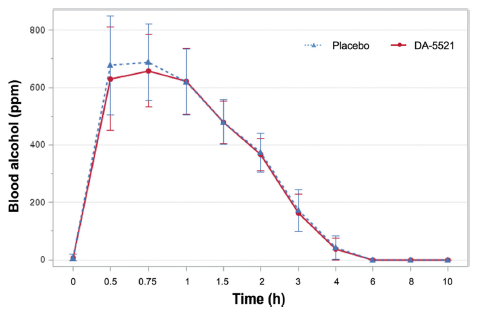
In this study, when participants consumed DA-5521, their blood alcohol concentrations at 0.5 and 0.75 h after alcohol intake were measured as 630.46±179.58 and 658.30±126.90 ppm, respectively. Conversely, when a placebo was ingested, the corresponding measurements were 677.38±171.70 and 688.65±132.28 ppm, respectively (Fig. 3). Compared to the ingestion of placebo, ingestion of DA-5521 resulted in a greater reduction in blood alcohol concentrations. Blood alcohol, acetaldehyde, and exhaled alcohol concentrations by group after alcohol intake are shown in Table 4. The area under the curve (AUC) for blood alcohol concentration was 1368.38±254.36 ppm·h when DA-5521 was administered and 1414.04±276.85 ppm·h when a placebo was administered, indicating no statistically significant difference between the two conditions. No statistically significant difference was observed in the time to reach the highest blood alcohol concentration (Tmax) which was 0.75±0.27 h when DA-5521 was ingested and 0.65±0.17 h when placebo was ingested. Furthermore, no statistically significant differences were observed in the maximum blood alcohol concentration (Cmax) which was 719.63±142.61 ppm following the ingestion of DA-5521 and 736.30±151.10 ppm following placebo ingestion. Several studies have explored the use of natural products to alleviate hangover symptoms following alcohol consumption (Kim et al., 2009;Lee et al., 2014;Kim et al., 2016). Bang et al. (2015) reported on the hangover-relieving potential of a polysaccharide-rich extract of Acanthopanax senticosus. The findings indicated that hangover symptoms (including headaches) were mitigated following alcohol consumption, however, no notable disparity was identified in blood alcohol concentration compared with the placebo group. Another study demonstrated the hangover improvement effect of an agent containing freeze-dried mature silkworm larval powder. The study demonstrated that the blood alcohol concentration decreased compared to the placebo group at 0.5 h after drinking. However, similar significant findings were not reported in the study by Woo et al. (2021).
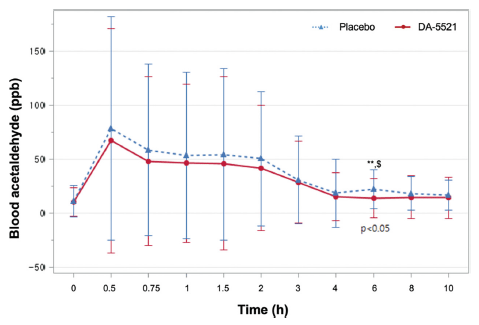
After alcohol consumption, either DA-5521 or a placebo was administered, and hourly measurements of both blood acetaldehyde and blood alcohol concentrations were obtained. At the 6 h mark following alcohol consumption, the blood acetaldehyde concentration was 14.14±18.04 ppb after ingestion of DA-5521 and 22.42±18.21 ppb after the ingestion of placebo, demonstrating a statistically significant difference (p = 0.0262**) (Fig. 4). The AUC for blood acetaldehyde was 234.82±297.96 ppb·h following the ingestion of DA-5521 and 287.78±306.86 ppb·h following placebo ingestion, exhibiting no statistically significant distinction between the two groups. Upon ingestion of DA-5521, the blood acetaldehyde levels exhibited a Tmax of 1.56±2.80 h and a Cmax of 74.89±101.40 ppb, while placebo ingestion resulted in a Tmax of 1.77±2.36 h and a Cmax of 87.39±104.34 ppb, and the difference was statistically significant (Table 4). In a study validating the efficacy of formulations constituting fermented soybean and yeast extracts for alleviating hangovers, a substantial reduction in blood acetaldehyde levels was observed at both 4 and 6 h post-drinking. These results are consistent with the findings presented in the study by Kim et al. (2020). During an investigation using a plant-based mixture, a notable decrease in blood acetaldehyde levels was observed in comparison with the placebo group at time points spanning 0.5, 1, 2, and 4 h post-consumption (Jung et al., 2023). Furthermore, a report indicated that the consumption of freeze-dried mature silkworm larval powder resulted in a significant reduction in blood acetaldehyde levels within the 0.5 to 2 h timeframe following alcohol consumption (Woo et al., 2021). Conversely, a study examining the effect of juice from the Korean pears (Pyrus pyrifolia cv. Shingo) displayed that the juice could influence the alcohol detoxification process post-consumption, however, it did not yield a statistically significant reduction in blood acetaldehyde levels compared to a placebo (Lee et al., 2013). These results displayed that DA-5521 significantly improved hangovers by reducing the concentration of acetaldehyde in the blood.
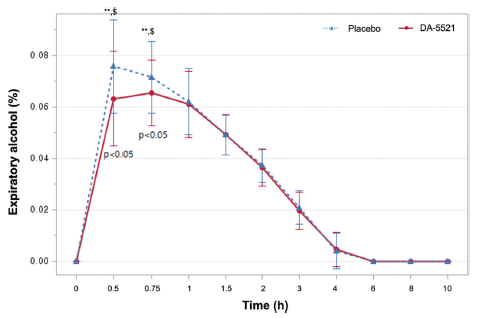
A small amount of 2-5% of the total alcohol intake by an individual is usually excreted from the body without major alterations in the breath, urine, and perspiration (Jones, 2006). Although breath alcohol concentration does not directly correlate with blood alcohol concentration, many countries have recognized breath alcohol concentration as admissible legal evidence (Jones, 2019). In this study, breath alcohol concentrations 0.5 h post-drinking was 0.0632±0.0185% following the ingestion of DA-5521, whereas it was 0.0758±0.0181% after placebo ingestion (p = 0.0177**). In addition, the breath alcohol concentration 0.75 h after drinking was 0.0655±0.0128 after DA-5521 and 0.0717±0.0139% after the placebo (p = 0.0310**) (Fig. 5). The AUC of breath alcohol concentration was 0.1414±0.0297%·h after DA-5521 ingestion and 0.1491±0.0324%·h after placebo ingestion. However, the values were not statistically significantly different. Following alcohol consumption, the ingestion of DA-5521 resulted in a Tmax of 0.6705±0.2097 h, while the ingestion of placebo yielded a Tmax of 0.6023±0.1258 h, with no statistically significant difference between the two groups. Moreover, Cmax demonstrated a statistically significant difference of 0.0721±0.0138% after DA-5521 ingestion following alcohol consumption and 0.0799±0.0168% after placebo ingestion (p = 0.0117**) (Table 4). One other study measuring breath alcohol concentrations after drinking displayed no statistically significant difference after consuming freeze-dried mature silkworm larval powder and a placebo. In contrast, another study demonstrated decreased breath alcohol concentration compared to placebo at 0.5 and 8 h (Woo et al., 2001). Additionally, when red ginseng is consumed after drinking, it has a positive effect on alleviating hangovers by significantly reducing breath alcohol concentrations at 0.5 and 1 h (p = 0.005 and 0.065, respectively) (Lee et al., 2014). Consumption of krill oil alleviates symptoms of thirst and nausea after a hangover, and the Cmax of breath alcohol exhibited a statistically significant difference of 0.07±0.01%. (Kim et al., 2022). The aforementioned findings are similar to the results observed in the current study.
For safety assessment, the analysis was conducted on participants who had ingested DA-5521 at least once after randomization in the trial (Safety Set). This study had a total of 24 participants, 12 of whom were allocated to the DP group and 12 to the PD group. No adverse reactions were observed in the DA-5521 group, however, one mild adverse reaction was observed in the placebo group, but the difference was not statistically significant (p = 1.0000). Clinical pathology tests were performed at visits one, two, and three. The assessment parameters were categorized into hematological, blood chemistry, and urine tests. In all parameters of the hematological test, no statistically significant differences were observed between the DA-5521 and placebo groups at 10 h post-drinking (Table 2). The findings indicated that alcohol consumption resulted in a significant increase in fluid intake and urine output accompanied by a reduction in urine specific gravity (Lawson, 1977). In this study, a statistically significant difference between the groups was observed in the urine specific gravity (p = 0.0277), however, the investigator deemed this difference clinically insignificant (data not shown). No statistically significant differences were observed in the other urine test parameters between the groups. Data pertaining to vital signs (blood pressure and pulse) and physical measurements (weight) are presented in Table 3. Typically, following alcohol consumption, an immediate increase in the pulse rate occurs followed by a decrease in blood pressure (acute depressor effect). However, blood pressure starts to rise (subacute pressor effect) after an 8-h period, and blood pressure returns to the previous levels after 24 h of sobriety (Arkwright et al., 1982;Kawano et al., 1992). In this study, the alteration in systolic blood pressure measured at the 2-h mark following alcohol consumption amounted to -8.52±8.17 mmHg in the DA-5521 group and -3.58±7.88 mmHg in the placebo group (p = 0.0405*). The alteration in diastolic blood pressure recorded at the 2 h post-drinking mark was -10.78±6.58 mmHg in the DA-5521 group and was statistically significantly different from blood pressure of -4.58±9.12 mmHg in the placebo group (p = 0.0108*). No statistically significant differences were observed between the groups for the other measured parameters.
In summary, the pre-consumption of DA-5521 among adults effectively alleviated hangover symptoms by diminishing blood acetaldehyde levels. Additionally, the safety evaluation outcomes suggested that the ingestion of DA-5521 is safe for human consumption.