Introduction
The Food and Agriculture Organization of the United Nations (FAO) has predicted that the population will increase to 10 billion by 2050, which will increase environmental pollution due to food and feed production increases (FAO, 2017). Edible insects have an eco-friendly advantage with fewer environmental pollution problems because they have lower gas emission such as carbon dioxide (CO2), methane (CH4), and nitrogen dioxide (N2O) than conventional livestock such as pigs and cattle known to have high reproductive power during being raised (Van Huis, 2016; Orsi et al., 2019). In addition, protein contents of edible insects range from 31% to 65%, higher than those of beans (23.5% of protein), lentils (26.7%), and soybeans (41.1%). Thus, edible insects are attracting attention as alternative protein sources for the future food (Gould & Wolf, 2018). As of 2021, ten edible insects including Batryticatus Bombyx, silkworms (larva, pupa), grasshoppers, Tenebrio molitor, Gryllus bimaculatus, Protaetia brevitarsis (larva), Allomyrina dichotomy, Zophobas morio, Apis mellifera, and Locusta migratoria have been recognized as food ingredients in Korea.
Among these ten edible insects in Korea, P. brevitarsis larvae is an insect with complete metamorphosis (eggs, larvae, pupa, and adults) and it belongs to the family of Coleoptera. It is widely distributed in Korea, Japan, Taiwan, China, and Europe (Chung et al., 2013). P. brevitarsis larvae contain about 17% carbohydrates, 58% proteins, and 18% fat. It has been reported that they possess various beneficial properties, including high-quality protein, antioxidant activity, antithrombotic efficacy, neuroinflammation-inhibitory effect, and liver function improving effect (Kwon et al., 2013; Lee et al., 2017; Choi et al., 2019; Lee et al., 2019).
Edible insects can easily become rancid during storage due to their high protein and lipid contents. Therefore, pretreatment methods are important. The Belgian Federal Food Safety Authority (FASFC) has stated that edible insects need heat treatment such as blanching to reduce the number of microorganisms (Vandeweyer et al., 2017). Methods of pretreatment and preservation of edible insects include roasting, frying, blanching, steaming, dipping, smoking, and baking. The blanching method is the most preferred pre-treatment method for various raw food stuff and edible insects. The heat of hot water can penetrate into insects and sacrifice them in a short time to prevent deterioration. It is an appropriate method for reducing microorganisms and foreign substances (Rumpold et al., 2014; Son et al., 2016; Dagostin, 2017). Sacrificing method is certainly a key step in insect processing. However, not enough research has been done and there are no specific regulations regarding the insect processing. (Caligiani et al., 2019; Larouche et al., 2019; Leni et al., 2019). Study on the black soldier fly, highlighted that Sacrificing method of prepupae impact the lipid composition. Furthermore, Larouche et al. (2019) and Singh et al. (2020) reported that sacrificing method has a significant influence on the final quality of insect. Longer sacrifice times induced stress, which is associated with increased metabolism of energy reserves (metabolism of triglycerides to acylglycerol and free fatty acids) and promotes oxidation (Caligiani et al., 2019).
As insects are prone to color change and oxidation of PUFA lipid fractions during processing, challenges remain to be addressed (Caligiani et al., 2019; Leni et al., 2019). There are several studies on methods of sacrificing insect. However, there are few studies assessed the effect of different sacrificing methods, defatting process, and storage method, on the quality of the final product. Base on this, we considered a sacrificing method that is industrially convenient and minimizes stress on inset. Therefore, in this study, live P. brevitarsis larvae were processed to produce P. brevitarsis larvae powder, by using blanching, freezing, storage temperature, and defatting treatment and investigated their effect on the quality characteristics of P. brevitarsis larvae powder.
Materials and Methods
Folin-Ciocalteau phenol reagent, 2,2–diphenyl–1–picrylhydrazyl radical (DPPH), gallic acid and L–ascorbic acid were purchased from Sigma Aldrich (St. Louis, MO, USA). All other chemicals used were of analytical grade.
The third instar larval stage of P. brevitarsis larvae were obtained from Jeju insect farming association corporation. P. brevitarsis larvae were fasted for 7 days to remove all rubbish and then they were washed before sacrificing. P. brevitarsis larvae were sacrificed by blanching (95 °C, 1 min) and quick freezing (-80 °C). And then they were vacuum packed and stored in a freezer at -80 °C or -20 °C. Stored P. brevitarsis larvae were hot-air dried at 60 °C (PS-100C, Shiniltech, Gimhae, Korea) until below 15±5% moisture contents.
Dried P. brevitarsis larvae were ground to a fine powder using a grinder (SHMF-3500G, Hanil, Seoul, Korea), it was pass through a 40–mesh sieve. The insect sample was defatted using n–hexane. A 1:5 ratio of hexane (w/v) was mixed with powder and stirred for 18 h. Every 6 hour, after filtration, Fresh hexane was added. Defatted samples were dried under a fume hood to evaporated remained hexane.
The appearance of the P. brevitarsis larvae on white plate was observed every process. The yield of the defatting and drying was calculated as a weight percentage before and after drying. The proximate composition of the powder was analyzed following the AOAC (1990) procedures.
The pH was measured in triplicate by vortexing 1 g of powder in 10 mL of distilled water. The pH was determined digital pH-meter (S 470 SevenExellence TM , Inti Inc., Schwerezenbach, Switzerland). The color was measured in triplicate with a chroma meter (CR-210, Minolta Co., Osaka, Japan). A standard plate (L=91.29 a=1.69 b=-12.86) was used as the standard.
Protein deterioration was observed by measuring volatile base nitrogen contents. The official method of the commission of the Korea Food and Drug Administration (Ministry of Food and Drug Safety, 2020) was applied. Briefly, 10 g of samples were stirred in 50 mL distilled water for 30 min and filtered. The samples were neutralized to weak acidity by adding 5% H2SO4. After addition of 1 mL of the above samples and 1 mL of saturated potassium carbonate solution to the outer chamber of the conway unit and add 1 mL of 0.01 N H2SO4 into the inner section. The lid was closed and incubated at a 25 °C for 1 h. Then titrated with 0.01 N NaOH. The VBN content was expressed in mg%.
The powder's total phenolic content was determined with the Folin-Ciocalteu method as described (Singleton & Rossi, 1965) with some modification. Briefly, 200 μL of the sample was mixed with 900 μL of distilled water and 100 μL 2 M Folin–Ciocalteu’s phenol reagent. The mixtures were kept in a darkness at room temperature for 5 min. Then, a 300 μL of 2% Na2CO3 and 500 μL of distilled water were added to the mixture, which was kept in darkness for 1 h. The absorbance at 760 nm of all samples was measured. Gallic acid was used as a standard.
The antioxidant activity of the powder was measured as described (Mensor et al., 2001) with some modification. Briefly, 2 mL of sample was mixed with 800 μL of 0.3 mM DPPH (in 95% ethanol). The mixtures were kept in a darkness at room temperature for 30 min. The absorbance at 517 nm of all samples was measured. Ascorbic acid was used as a standard. The radical scavenging activity (%) was calculated by the following formula:
Minitab 18 (Minitab Inc., State College, PA, USA) was used. All experiments were carried out in triplicate and results were reported as the mean ± standard deviation. Significant differences (p<0.05) between treatments were determined with One way ANOVA, followed by Tukey and an independentsamples T-test was used to analyze the mean comparison according to defatting treatment.
Results and Discuusion
The appearance of P. brevitarsis larvae is shown in Fig. 1. In general, these larvae crawl around using their back because their legs are not developed. It was found that each body segment of the larvae had hairs. The growth of P. brevitarsis larvae could be divided in first instar, second instar, and third instar larval stages. Sizes of P. brevitarsis larvae were 0.3-20 mm, 25-30 mm, and 40-45 mm, respectively during growing. Third instar larvae of P. brevitarsis larvae were used in this study. The color of blanched larvae was whiter than that of larvae without the blanching treatment. Insect browning can occur due to enzymatic reactions caused by enzymes such as tyrosinase, tyrosine hydroxylase, L-DOPA decarboxylase, laccase, and peroxidase (Singh et al., 2020) or non–enzymatic reactions in which amino acids and reducing sugars can react with each other to produce melanoidins (David-Birman et al., 2018). It is possible to use blanching method to sacrifice the larva in a short time by applying high heat to the larva. However, a quick-freezing method takes a long time to completely sacrifice insects due to metabolic heat (Cooper et al., 1984). For this reason, non-heating sacrificial methods such as quick freezing do not inactivate enzymes or microorganisms in the larvae, resulting in a darker color of the final product. According to Leni et al. (2019) black soldier fly (BSF) killed by freezing shows a darker color than blanched BSF. In the present study, blanching was also effective in prevent browning. All larvae pretreated by the three methods (S1: blanched at 95 °C and stored at -20 °C, S2: blanched at 95 °C and stored at -80 °C, S3: frozen at -80 °C and stored at -80 °C) did not maintain their original appearance after hot air drying. They were deformed because of rapid evaporation of moisture.

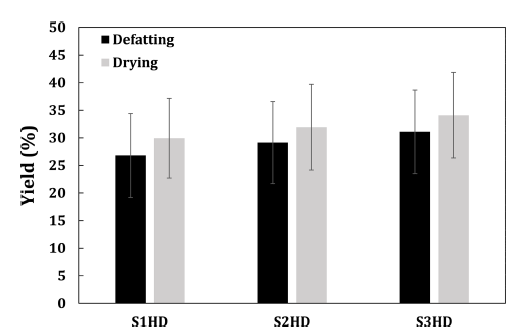
Defatting yield and drying yield of P. brevitarsis larvae powder processed under various conditions are shown Fig. 2. There was no significant (p>0.05) difference in the defatting yield of powder obtained by different sacrificial methods and storage temperatures. In the case of protein foods with high fat contents, the defatting process can directly affect protein recovery and purity of the final product. P. brevitarsis larvae have high fat contents, so if they are not defatting process, the powder will aggregate. In addition, its yield will decrease, and fat oxidation can occur during storage. Therefore, a defatting process is needed to improve the storage stability of the powder.
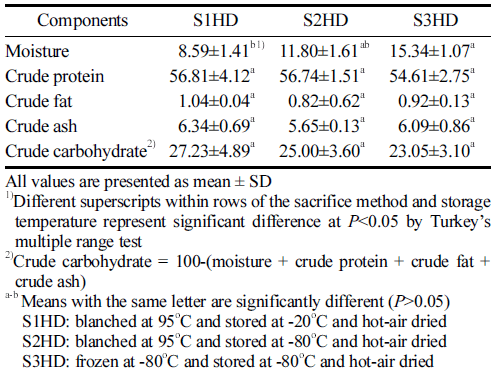
The proximate composition of P. brevitarsis larvae powder samples obtained with a defatting process and different sacrificial methods are summarized in Table 1. Previous studies on nutrient composition (% Dry Matter Basis) of P. brevitarsis larvae have indicated that they have abundant nutrients, including crude protein content ranging from 44.23% to 66.65%, crude fat content ranging from 10.40% to 26.81%, and crude ash content ranging from 4.45% to 8.45% (Yeo et al., 2013; Back et al., 2017;Kim et al., 2020; Lee et al., 2020). In general, neither sacrificial method seemed to have any significant effect on the proximate composition of P. brevitarsis larvae powder in the present study. S1HD, S2HD, and S3HD showed moisture contents of 8.59%, 11.80%, and 15.34%, respectively. In this study, we obtained average moisture content and fat contents of 78.07 g/100 g and 3.54 g/100 g fresh weight of P. brevitarsis larvae before drying, respectively. After drying and defatting processes, the moisture content was between 8.15% and 15.34% and the fat content was between 0.82% and 1.04%. Lipid oxidation stability of P. brevitarsis larvae could be improved by reducing the fat content. There was no significant (p>0.05) difference in protein, fat, ash, or carbohydrate content according the processing condition. Defatted powder had protein content of 54.61%-56.81%. This content was much higher than those found for eggs, meat, and fish. Highlevel proteins might improve the functionality of the powder (Kim et al., 2021). In this study, P. brevitarsis larvae showed high protein contents independently from the sacrificial method. This suggests that P. brevitarsis larvae are highly valuable as a food ingredient. The WHO/FAO has explained that a food can be called protein-rich if its protein content is 10 g/100 g edible part (Udomsil et al., 2019).
|
The crude ash content of defatted P. brevitarsis larvae was 5.09% to 6.34%, which was similar to or lower than results of other studies (5.72% to 12.09%) (Kim et al., 2020;Kim et al., 2021). However, Ghosh (2017) has reported that five edible insects have higher mineral contents than conventional foods of animal origin, and P. brevitarsis larvae having the highest iron contents among minerals.
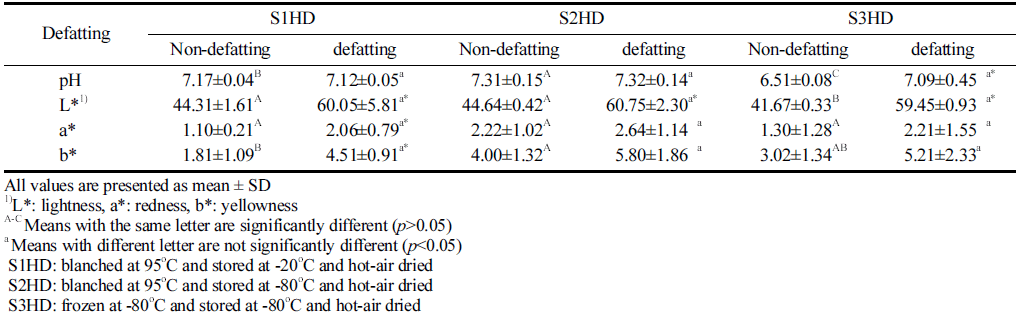
Results of pH and color of P. brevitarsis larvae powder processed under various conditions are shown Table 2. The pH of animal food is an important indicator for a change in its properties during storage. The pH values of P. brevitarsis larvae powder obtained with different sacrificial methods and a defatting process were found to be between 6.51 and 7.32. The pH of the S3HD sample was significantly (p<0.05) lower than that of S1HD of S2HD before defatting. This might be due to glycosis. Sanchez-Paz et al. (2006) have shown that insects, like invertebrates, can keep energy reserves in the form of glycogen. Some previous studies using stressed (exhaustion) invertebrates in the field of culinary have shown low pH values (Ellington, 1983; Hervant et al., 1999). This phenomenon is due to accumulation of lactic acid because of glycosis. In this study, S1HD and S2HD were killed immediately by hot-water blanching. However, S3HD took a relatively longer time to die under -80 °C. Thus, P. brevitarsis larvae might have been stressed and glycolysis might have occurred during the freezing process. Farina (2017) has reported that the pH of cooked broth made from live insects is lower than that made from frozen insects, and it is consistent with the findings of the present study. The S3HD showed a significantly higher pH after a defatting process. This was generated that the increase in pH is correlated with mineral content. Kim et al. (2019) also reported that contents of minerals such as potassium (K), phosphorus (P), and magnesium (Mg) were higher in Zophobas atratus larvae after defatting than those before defatting. The pH of insect powder might change depend on the sacrificial method; however, it showed no significant difference according to the sacrificial method due to an increase in mineral content after the defatting process.
|
The lightness of P. brevitarsis larvae powder after defatting was higher (p<0.05) than that before defatting, consistent with results of Son et al. (2019). They observed that the lightness value of defatted Tenebrio molitor with n-hexane was the highest. Mishyna et al. (2019) have also reported that defatted insect powder has a higher lightness due to fat removal. Kan et al. (2008) have analyzed pigment produced by arthropods and found that phenol oxidase can promote the production of quinone and form melanin pigment. Thus, the lightness might have been increased by eluting the melanin pigment of insects during the defatting process. And the lightness improvement has a positive influence on the consumer preference. Meanwhile, there was no significant (p>0.05) difference in the redness value according to the type of Sacrificing method before or after defatting. Dye produced by the arthropods were that the phenol oxidizing enzyme (Phenol oxidase) that promotes the formation of the quinone (Quinone) to form a melanin pigment.
The VBN value is an important indicator to evaluate the freshness of an animal product. The VBN is a generic term of volatile, basic nitrogen-containing substances such as ammonia, primary amines, and secondary amines produced through decomposition of proteins by enzymatic reactions and bacteria. The quality of insects with high protein contents can be influenced by protein deterioration. According to the Korean Food Standards Codex, the VBN in fresh meat should be under 20 mg%. The VBN of P. brevitarsis larvae powder according to the sacrificial method and defatting process in the present study was below 20 mg%. In the case of insects, it is difficult to apply the standards for fresh meat. However, this was carried out to confirm the tendency of protein deterioration according to pretreatment. Results of VBN of P. brevitarsis larvae processed under various conditions are shown in Fig. 3. After defatting, there was a significant (p<0.05) difference in VBN of P. brevitarsis larvae powder according to the pre-treatment method. Due to the difference in moisture content of the raw material according to the sacrificial method, S3HD took 15 h to dry, while S1HD and S2HD only took 12 h to dry. For this reason, a higher decomposition of protein was expected for P. brevitarsis larvae powder obtained with freezing as a sacrificial method. In addition, the VBN of S2HD was higher than that of S1HD probably due to a higher moisture in S2HD. Proteins are associated with lipids in food structures and membranes. Thus, protein co-oxidation is strongly interconnected with lipid oxidation in protein products. Indeed, protein could react with most intermediate and final products from lipid oxidation. The lower the moisture, the slower the rate of co-oxidation (Schaich, 2013;Estevez, 2011; Tournour et al., 2017). Conversely, Singh et al. (2020) have observed that thermal treatment such as blanching can lead to the highest protein and lipid oxidation, which is also increased with longer storage period. Quick freezing method resulted in a lower initial oxidation of the powder which did not increase with storage period. The difference trend from this study might be due to different conditions of blanching used in this study.
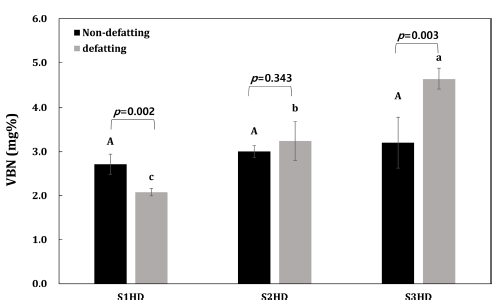
In addition, between before and after defatting of each sample, results were compared using two-sample t-test (p<0.05). Results of the experiment showed that the defatting process affected protein deterioration in S1HD and S3HD. The protein deterioration of P. brevitarsis larvae powder might be due to differences in components after defatting. Food proteins are sensitive to oxidative reactions, leading to possible degradation of food properties such as lower digestibility (Estévez, 2011). Results of the present study suggest that defatted S1HD exhibits lower VBN value, thus having a potential for food applications.
Total phenol content is commonly used to indicate antioxidant levels. Phenolic compounds are secondary metabolites present in the plant world. They can be used as an indirect indicator of antioxidant activity. Total phenolic contents of P. brevitarsis larvae processed under various conditions are shown Table 3. Total phenol content of P. brevitarsis larvae sacrificed by freezing at -80 °C was higher (p<0.05) than that by blanching. Polyphenol component of P. brevitarsis larvae was destroyed by blanching heat. Defatted P. brevitarsis larvae powder showed higher phenol content than the non-defatted powder.
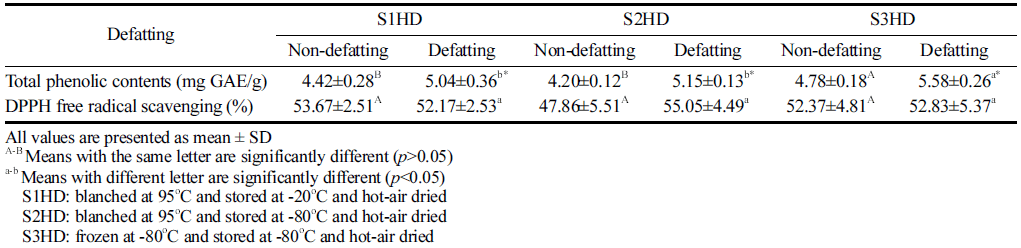
|
The protein content of defatted P. brevitarsis larva powder was higher than that of non-defatted larvae powder. Thus, it would be easy for defatted powder containing high protein content to bind to polyphenols. Suh & Kang (2011) have indicated that the feeding trait is responsible for phytochemical compounds in P. brevitarsis larvae. In the present study, P. brevitarsis sacrificed by freezing at -80 °C showed the highest total phenolic content. Further study is needed to determine the association between protein level and polyphenol component.
To measure the antioxidant activity, DPPH radical scavenging activity assay is a widely utilized. Results of DPPH radical scavenging activity of larvae powder samples obtained with various pretreatment conditions are shown Table 3. There was no significant difference (p>0.05) in DPPH radical scavenging activity (47.86-55.05%) among samples. All powder samples after a defatting process showed antioxidant activities. Most of them showed the DPPH radical scavenging activity of 52.17% to 55.05% (p>0.05). Singh et al. (2020) have also reported that DPPH radical scavenging activities of crickets sacrificed by the blanching method and the freezing method at -20 °C followed by hot air drying are 60.4% and 59.6%, respectively, showing no significant (p>0.05) difference between the two. Therefore, the type of sacrificial method and defatting process did not affect the DPPH radical scavenging activity.
From all results of this study, it indicated that S1HD (blanching, defatting, -20 °C storage) was an optimal processing method for producing Protaetia brevitarsis larvae powder. Blanching can secure food safety by inactivating enzymes and preventing protein deterioration as much as possible by reducing drying time in terms of quality. The combination of blanching and a defatting process can improve the storage stability by removing lipid. A defatting process is an essential process for extending the shelf life and securing the food safety of edible insects rich in lipids. At -20 °C storage would be more economical by preventing protein deterioration, although low temperature (-80 °C) effectively maintained antioxidant activity components of insect powder. Moreover, it was suitable to have more proteins in dried food.
Conclusion
Effects of various pre-treatment methods (blanching, quickfreezing, storage temperature, defatting treatment) on characteristics of Protaetia brevitarsis larvae were investigated. Results of this study indicated that S1HD (blanching, defatting, -20 °C storage) was an optimal processing method for producing Protaetia brevitarsis larvae powder. Blanching can secure food safety by inactivating enzymes and preventing protein deterioration as much as possible by reducing drying time in terms of quality. Moreover, a low temperature of -80 °C is suitable to maintain antioxidant activity components. However, to have more proteins in dried food, the combination of blanching and -20 °C storage would be more economical by preventing protein deterioration. A defatting process can improve the storage stability by removing lipid. A defatting process is an important process for extending the shelf life and securing the food safety of edible insects rich in lipids.








