Introduction
Salicornia europaea L. (SE), commonly known as glasswort, is a halophyte in the family Amaranthaceae with the highest salt tolerance under more than 1 M NaCl. It is widespread in the salt-affected environments including tidal floodways, brine springs, and salt lakes, with in particular being abundantly in coastal regions of East Asia (Kang et al., 2015;Panth et al., 2016). It is commonly consumed as a raw vegetable for salad, food ingredient, and a fermented food in European countries and Korea (Seo et al., 2010;Patel, 2016). In addition, there have been accumulating studies reporting its therapeutic potentials as antioxidative, anti-inflammatory, antihypertensive, antimicrobial, anti-tumor, anti-diabetic, antihyperlipidemic, and hepatoprotective activities (Kim et al., 2009;Cho et al., 2016). Although SE has diverse biological activities, there could be a limit to the direct applications of SE itself due to high concentration of salt in SE. The intake of high salt is one of major risk factors in the developments of hypertension and hypernatremia and the induction of apoptotic cell death (Michea et al., 2000;Kim, 2006;Drenjančević-Perić et al., 2011). Therefore, the desalination strategies have been developed including distillation, electrodialysis, vapor compression, and reverse osmosis to reduce the dissolved salt content of the halophytes (Al-Amshawee et al., 2020). Focusing on the desalination of SE, there have been several studies on the reinforced functionality of desalted SE (DSE) (Yoon et al., 2017), the attenuation of visceral adipocyte by DSE (Rahman et al., 2018), and the anti-neuroinflammatory and anti-amnesic effects of DSE (Karthivashan et al., 2018). In addition, as a lot of interest has arisen in the fermentation of plants using microorganisms, the studies on microbial fermentation of SE have been reported; the antioxidative and fibrinolytic activities of SE fermented by Lactobacillus acidophilus (Song et al., 2007), the angiotensin converting enzyme inhibitory activity of SE fermented by Bacillus subtilis (Park et al., 2009) and Aspergillus oryzae (Kim et al., 2010).
1-Deoxynojirimycin (1-DNJ) as representative iminopyranose alkaloid is one of well-known α-glucosidase inhibitors and was firstly isolated from the root bark of a mulberry tree as being named to be moranoline (Yagi et al., 1976). It has been reported to have a role in treating various metabolic diseases with antiviral, antitumor, anti-obesity, antioxidative, and antiinflammatory activities, which enabled to be applied for the developments of functional food materials and therapeutic agents (Shibano et al., 2008;Park et al., 2013;Gao et al., 2016;E et al., 2017). The natural sources for 1-DNJ production has been reported to be plants (including Morus alba L., Commenlina communis, Hyacinthus orientalis), insects (including Bombyx mori silkworms), and microorganisms (including Streptomyces lavendulae and Bacillus subtilis) (Zhang et al., 2019). Among these strategies for 1-DNJ production, microbial sources have been recently attracted with increasing attention due to fast growth rate, high production yield, and low cultivation cost (Zhang et al., 2019). However, the studies on microbial producers of 1-DNJ have been largely limited to several strains of Streptomyces sp. and Bacillus sp.; S. lavendulae (Ezure et al., 1988), S. subrutilis (Hardick et al., 1992), B. subtilis (Stein et al., 1984), B. amyloliquefaciens (Seo et al., 2013), and B. atrophaeus (Jiang et al., 2015). Previously, we had isolated B. velezensis K26 producing 1-DNJ from Korean fermented soybean paste (Doenjang) and analyzed its genome to functionally mine 1- DNJ biosynthetic gene cluster (Lee et al., 2018;Lee et al., 2021). Although the microbial 1-DNJ producers have been isolated and its α-glucosidase inhibitory activity has been steadily evaluated, relatively few studies have been reported regarding the production of functional fermented foods or the related biomaterials capable of producing 1-DNJ. These previous studies included the Chinese traditional okara (soy pulp) fermented by B. subtilis (Zhu et al., 2010), Korean soybean paste fermented by B. amyloliquefaciens (Seo et al., 2013), and mulberry leaf extract fermented by Lactobacillus plantarum (Choi et al., 2020), reporting the α-glucosidase inhibitory activity of fermented foods, of which responsibility could be 1-DNJ. However, there was no studies on the fermentation of DSE (even SE) with the microbial 1-DNJ producer focusing on the anti-obesity and anti-inflammatory activities, even though all of DSE and 1-DNJ have been reported to confer these biological activities. In this study, we investigated the production of 1-DNJ and the synergistic activity of α-glucosidase inhibition from the DSE fermented by food (Doenjang)-derived B. velezensis K26 (BVDSE), which had been previously identified to produce 1-DNJ (Lee et al., 2018). In addition, we examined the anti-obesity and anti-inflammatory activities of BVDSE in 3T3-L1 adipocytes and RAW264.7 cells, respectively, to support the potential application of BVDSE as functional fermented food or biomaterial.
Materials and Methods
Desalted Salicornia europaea L. (DSE) was provided by the R&D center of Phyto Corporation (Seoul National University, Seoul, Korea). Bacillus velezensis K26 and B. subtilis 142, isolated from Korean fermented soybean paste (Doenjang) (Lee et al., 2018), were used to ferment DSE as being considered to be 1-DNJ producer and negative control strain (non-producer for 1-DNJ), respectively. These strains were sub-cultured in 5 mL of tryptic soy broth (TSB, Difco, BD Biosciences, Franklin Lakes, NJ, USA) at 37 °C for 24 h and then inoculated into DSE, followed by being incubated at 37 °C for 5 ds. The nutritional analyses of the products fermented in only DSE itself and DSE with B. velezensis K26 (BVDSE) were conducted and by the Korea Health Supplement Association (KHSA, Seongnam, Korea).
α-Glucosidase inhibitory (AGI) activity of DSE, the fermented DSE with B. velezensis K26 (BVDSE), and with B. subtilis 142 (BSDSE) was determined according to our previous method (Lee et al., 2018). Briefly, each cell-free sample was prepared by centrifuging the fermented broth at 10,000 rpm for 10 min. The sample (147 μL) was then mixed in 100 mM phosphate buffer (pH 7.2, 30 μL) with 10 mM p-nitrophenyl α-glucopyranoside (30 μL, pNPG, Sigma-Aldrich, St. Louis, MO, USA). The addition of 100 U/mL α-glucosidase (3 μL, Sigma-Aldrich) in the mixture initiated the reaction of which incubation continued at 37ºC for 10 min. The enzyme reaction was then quenched by adding 500 mM Na2CO3 (100 μL). Finally, the absorbance of p-nitrophenol was measured at 405 nm using a Multiskan FC spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The AGI activity was calculated using the following equation.
1-DNJ production in the fermented BVDSE was monitored by ultra-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight–mass spectrometry (UPLCESI- Q-TOF-MS) as previously described (Lee et al., 2018). Briefly, the cell-free sample prepared as above mentioned was extracted with methanol (1:1, v/v) and then filtered using a 0.22-μm polyvinylidene difluoride filter. Thereafter, 1-DNJ from the prepared sample was analyzed using Agilent 1290 Infinity UPLC system with Agilent 6520 Q-TOF mass spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Separation was carried out on a hydrophilic interaction LC (HILIC) column (ZORBAX HILIC Plus, 2.1 × 100 mm, 3.5 μm; Agilent Technologies). The flow rate and injection volume were 0.3 mL/min and 1 μL, respectively. The mobile phase consisted of 5 mM ammonium acetate in water (A) and 0.1% formic acid in acetonitrile (B). The following UPLC gradient elution conditions were used: 90% B at 0 min, 90% B at 0.1 min, 60% B at 9.9 min, 100% B at 1 min, 100% B at 2 min, 90% B at 1 min, and 90% B at 6 min. The N2 gas flow rate was 8 L/min at a temperature of 325 °C. The fragment and capillary voltages were set at 70 V and 4000 V, respectively. The authentic 1-DNJ compound was obtained from Sigma- Aldrich and considered to be a standard compound.
3T3-L1 mouse pre-adipocytes (ATCC, Manassas, VA, USA) were seeded in a 48-well plate and cultured in DMEM (Dulbecco’s Modified Eagle’s Medium, Cambrex BioScience, Walkersville MD, USA) containing 10% bovine calf serum (BCS) and 1% penicillin (100 U/mL)/streptomycin (100 μg/ mL) (PS) at 37 °C in 5% CO2 for 2 d. After confluence, 3T3-L1 cells were stimulated to induce differentiation in high-glucose DMEM with 10% FBS, 1% PS containing MDI (0.25 μM dexamethasone, 0.5 mM isobutylmethylxanthine, 10 μg/mL insulin). After 48 h, the differentiation medium was changed to DMEM-insulin (DMEM, 10% FBS, 1% PS, 10 μg/mL insulin). Two days later, the 3T3-L1 cells were grown in a DMEM-normal medium (DMEM, 10% FBS, 1% PS), and a DMEM-normal medium was exchanged every 2 d for 6 d. BVDSE was treated to identify its effect on adipocyte differentiation with DMEM-MDI, DMEM-insulin, and DMEMnormal medium for 10 d after confluent.
The mouse macrophage RAW 264.7 cells were purchased from Korea Cell Line Bank (KCLB, Seoul, Korea). The cells were cultured in DMEM (Hyclone, Logan, UT, USA) added with 1% antibiotic/antimycotic solution (10 mg/mL streptomycin, 10,000 U/mL penicillin G, 25 μg/mL amphotericin B) (Hyclone, Logan, UT, USA) and 10% fetal bovine serum (FBS) at 37 °C in 5% CO2 incubator. The RAW 264.7 cells were then plated in a 24-well plate at 1×105 cells/mL. The samples were diluted 100-fold and treated on the cells for 24 h with DMEM. Thereafter, the cells were induced with 500 ng/mL lipopolysaccharide (LPS, Sigma-Aldrich, St. Louis, MO, USA) for 12 h. Positive and negative controls were considered to be treated with only 500 ng/mL LPS without sample and not treated, respectively.
The water-soluble tetrazolium salts (WST-1) cell viability assay (CellVia, Abfrontier, Seoul, Korea) was performed according to the manufacturer’s instructions. The 2×10 4 cells / well were seeded on 96-well plates and incubated for 24 h at 37 °C with 5% CO2. The samples were treated for 1, 5, 10, and 25% concentration for 48 h in 3T3-L1 cells. After 48 hincubation, 10 μL of CellVia reagent was added to the cells and incubated for 30 min at 37 °C. The absorbance was measured using a microplate reader (MultiSkan FC, Thermo Fisher Scientific, USA) at 450 nm.
The effect of BVDSE on adipocyte formation was investigated by Oil Red O staining using an adipogenesis assay kit (Enzo Life Sciences, Farmingdale, NY, USA). In a 48-well plate, 0.014×10 6 cells/well were cultured with the sample and the medium was then removed entirely. The cells were fixed with 100 μL of cell fixative solution at room temperature for 30 min and then stained with the filtered 0.3% Oil red O (w/v, Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 30 min, followed by being washed three times with deionized water. The stained cells were observed with a microscopic image. For quantification of lipid accumulation, the cells were extracted by 100 μL of extraction solution at room temperature for 30 min and the absorbance was finally measured at 490 nm.
The cells were harvested by centrifugation at 300 g for 5 min. The IL-6 and TNF-α in the culture supernatants were analyzed using ELISA kit (eBioscience, San Diego, CA, USA). A 96-well plate was coated with a capture antibody at 4 °C for 12 h, and 100 μL of sample diluent solution (ELISA kits) diluted with 100-fold was added and stood at room temperature for 1 h. After washing with cold-PBS (phosphatebuffered saline, Sigma-Aldrich), 100 μL of capture antibody was added and incubated for 1 h. Afterward, the biotinconjugated detection antibody mixed with SAv-HRP (streptavidin-conjugated horseradish peroxidase) was added. To induce a color reaction, 100 μL of substrate solution was added to react for 30 min and a stop solution (1 M H5PO4) was added to terminate the reaction. The cytokines in the sample were finally measured by absorbance using a microplate reader (Bio-Rad, Hercules, CA, USA) and quantified using a standard curve.
All statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad software, San Diego, CA, USA). Cell viability was analyzed by Two-Way ANOVA followed by Bonferroni posttest. Lipid accumulation and cytokine production containing IL-6 and TNF-α were analyzed by One-Way ANOVA with Tukey’s multiple comparisons test.
Results and Discussion
B. velezensis K26 as 1-DNJ producer, which had been previously isolated from Korean fermented soybean paste (Doenjang) in our study (Lee et al., 2018), was used to ferment DSE. First, we determined the AGI activity of BVDSE to easily monitor if the fermentation of DSE with B. velezensis K26 was well done, because 1-DNJ has been commonly known to be an α-glucosidase inhibitor. The AGI activity of BVDSE was determined to be 66.4% which was almost 2-fold higher than that of DSE (34.7%) (data not shown). The AGI activity of DSE itself had been already reported in the previous study with its other biological functionalities including antioxidant and anti-hypertensive activities (Yoon et al., 2017). Therefore, the enhancement of AGI activity in BVDSE could be supposed due to the production of 1-DNJ in B. velezensis K26 during the fermentation of DSE. To investigate the production of 1-DNJ in BVDSE, the UPLC-ESI-Q-TOF-MS analysis was conducted, resulted that a positive electro-spray ionization mass analysis of the BVDSE sample yielded a major molecular ion (m/z for [M+H]+ ) of 164.09 for 1-DNJ (C6H13NO4, molecular weight 163.17) (Fig. 1A). This peak was identical to the authentic 1-DNJ standard compound (Fig. 1B). These results suggested that the 1-DNJ could be well produced in the fermentation of DSE by B. velezensis K26 and its production increased the AGI activity in the BVDSE sample accordingly.

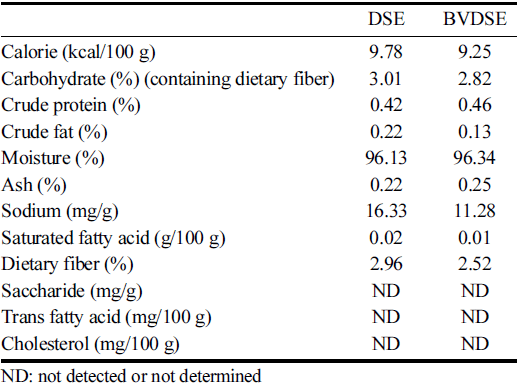
The changes in nutrient compositions of DSE-fermented products with and without B. velezensis K26 were shown in Table 1. The fermentation of DSE by B. velezensis K26 increased the crude protein, moisture, and ash contents compared to that of DSE itself, however, the changes seemed to be not significant. On the contrary, the carbohydrate, saturated fatty acid, and dietary fiber contents slightly decreased in the BVDSE sample. Compared to the DSE sample, the contents of crude fat and sodium in BVDSE significantly decreased to 0.13% and 11.28 mg/g, respectively. The decreases of crude fat and carbohydrate contents by microbial fermentation were consistent with the previous study reporting similar trends in the fermentation of Salicornia herbacea L. with B. subtilis KCCM 12247 (Park et al., 2009).
|
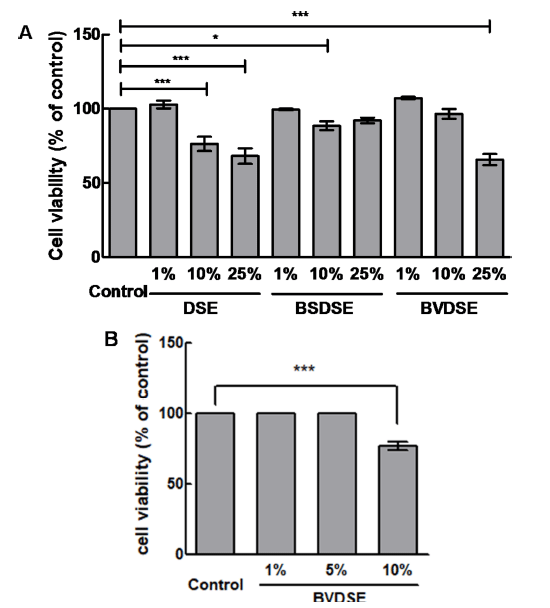
We examined the effect of BVDSE on the inhibition of adipocyte differentiation. In this experiment, we added the other DSE-fermentation sample fermented with B. subtilis 142 (BSDSE), a non-1-DNJ producer, as a negative control to evaluate if the reduction in lipid droplet formation and accumulation are due to microbial fermentation of the strain capable of producing 1-DNJ. The 3T3-L1 pre-adipocytes were treated with various concentrations of DSE, BSDSE, and BVDSE (1, 10, and 25%) for 48 h, and cell toxicity was then measured. There was no toxicity of all samples to 3T3-L1 cells at the concentration of 1% (Fig. 2A). When the sample was treated with 10% concentration, the cell viability for the DSE significantly decreased to 76.39±2.77%, while those for the BSDSE and BVDSE samples reached 88.53±1.70% and 96.55±1.86%, respectively. In the treatment at 25% concentration, the DSE and BVDSE samples led to the highly toxic effects on 3T3-L1 cells, showing the cell viabilities of 68.13 ±2.97% and 65.77±2.17%. However, there was no difference in cell viability for the BSDSE sample up to 25%. Nevertheless, we re-evaluated the cell viability of BVDSE at concentrations up to 10% (1, 5, and 10%), because our target sample is BVDSE, not BSDSE focusing on 1-DNJ producer. As the results, no significant effect on cell viabilities for BVDSE sample was observed under the treatment of 5% concentration (Fig. 2B). Therefore, further study on the inhibition effect of lipid accumulation was investigated by the treatment with 5% concentration of samples.
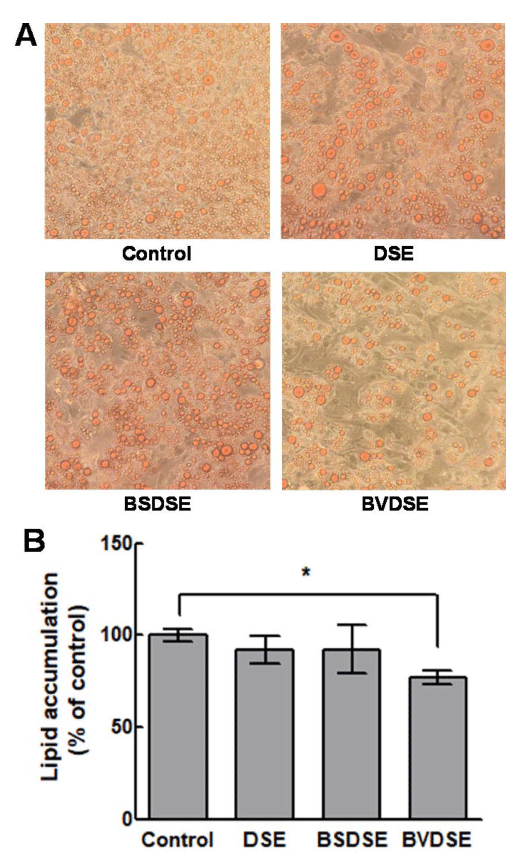
We examined the effects of DSE, BSDSE, and BVDSE on lipid accumulation of differentiated 3T3-L1 adipocytes by the microscopic examination of Oil Red O-stained lipid droplets (Fig. 3A). Oil Red O reagent only stains triglycerides and cholesterol esters, excluding free fatty acids and phospholipids, and is suitable for evaluating the lipid differentiation because most of the lipids accumulated by the fat differentiation are triglycerides (McNeel and Mersmann, 2003). The result demonstrated that BVDSE (5% treatment) significantly decreased Oil Red O-stained lipid droplet compared to the control sample which was not treated (Fig. 3A). On the other hand, DSE and BSDSE samples did not affect adipogenesis compared to BVDSE observed from the microscopic analysis. Therefore, the BVDSE sample was confirmed to reduce lipid accumulation by inhibiting the differentiation from 3T3-L1 pre-adipocyte to adipocyte. These results were confirmed by the quantification analysis of lipid accumulation, exhibiting that there were no significant inhibitions of lipid accumulation for the treatments of DSE and BSDSE samples compared to the control (Fig. 3B). However, BVDSE sample effectively inhibited lipid accumulation, in which lipid accumulation decreased about 23% compared that in the differentiated cells. The in vitro anti-adipogenic effects are associated with the in vivo anti-obesity effects (Meydani and Hasan, 2010). 1-DNJ isolated from B. subtilis MORI had been proven to have the protective effect against obesity-induced hepatic lipid abnormalities and mitochondrial dysfunction in the animal experiments using high-fat-fed mice (Do et al., 2015). 1-DNJ from mulberry has been reported to stimulate the secretion of adiponectin, which improves lipid metabolism in the dietinduced obese mouse model and murine 3T3-L1 adipocyte, by the expression of the adipogenic genes C/EBPα, PPAR-γ, and aP2 (Naowaboot et al., 2012;Tsuduki et al., 2013). Salicornia herbacea L. water extracts (SLW) have been reported to significantly increase the production of TNF-α in 3T3-L1 adipocyte, decrease the production of leptin and resistin associated with insulin resistance, and increase the production of PPAR-γ and adiponectin, which increases insulin sensitivity (Kim et al., 2014). Furthermore, when desalted Salicornia europaea powder (DSP) was administered to HFD (highinduced obese mice), the adipocyte-specific transcription regulators C/EBPα, SREBP1, FAS, and PPAR-γ were downregulated by DSP-derived TFA (trans-ferulic acids) (Rahman et al., 2018). Our results concluded that the anti-obesity effect of BVDSE was caused by 1-DNJ produced by B. velezensis K26 in the fermentation of DSE. Therefore, it is necessary to investigate the changes in the expression of mRNA in several adipogenic markers (PPAR-γ, C/EBPα) or termination markers (GLUT4, adiponectin) that play an important role in the differentiation of 3T3-L1 adipocytes by 1-DNJ.
The gene expression of TNF-α and IL-6 in LPS-induced RAW 264.7 cells upon treatment with DSE, BSDSE, and BVDSE samples are shown in Fig. 4. As a result of examining the secretion of the inflammatory cytokine TNF-α, the expression level of TNF-α of DSE was significantly higher than that in the non-treated group (Fig. 4A). Although BSDSE and BVDSE samples showed no significant change of the gene expression of TNF-α compared to non-treated group, their levels were lower than the level for DSE sample. The TNF-α as a pro-inflammatory cytokine initiates the pro-inflammatory cascades as activating inflammatory cells for recovery purposes. However, the over-expression of TNF-α could trigger chronic inflammation and further cell apoptosis (Brown et al., 2007). In fact, Salicornia herbacea L. had been previously reported to suppress LPS-induced nitric oxide production and the cytokine expressions including TNF-α and IL-1β in RAW 264.7 cells due to the presence of 3-O-β-d-glucopyranoside in Salicornia herbacea L. (Kim et al., 2009), which was opposite to our result regarding the activation of TNF-α expression in DSE sample. In view of the differing results, there may be two reasons; the source and the desalting processing of SE.
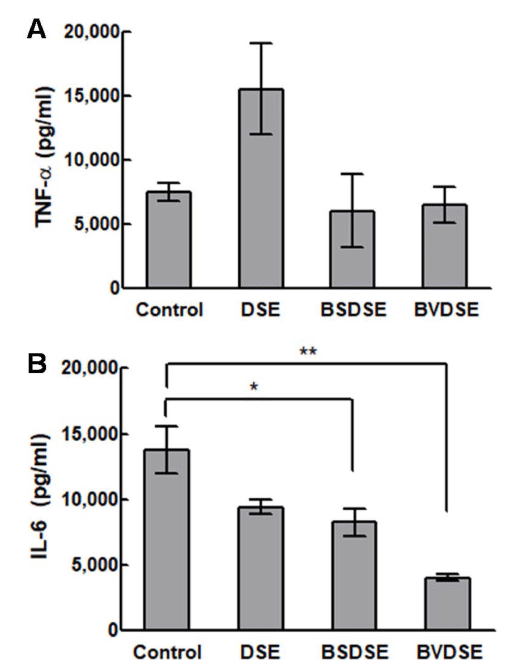
Next, we verified the effect of microbial fermentation of DSE on pro-inflammatory gene, IL-6 in RAW 264.7 cells induced with LPS. The DSE and BSDSE samples significantly suppressed (p < 0.05) the gene expression of IL-6, while the BVDSE sample more significantly inhibited (p < 0.01) LPSinduced IL-6 (Fig. 4B). IL-6 is produced by various cells stimulated by TNF-α, IL-1, and INF-γ, being involved in the regulation of various aspects in inflammation process (Ma et al., 2018). Therefore, IL-6 is strongly associated with TNF-α (Wang et al., 2017). However, our results showed that all fermented samples were able to inhibit the LPS-induced upregulation of IL-6, even though TNF-α did not show similar result. Finally, it could be noted that the communication between TNF-α- and IL-6-mediated inflammation in this study might be weak, possibly since the circulating levels may be affected by a number of different factors (Stenvinkel et al., 2005). Although the exact anti-inflammatory mechanism of BVDSE remains unclear, the mechanism could be elucidated in further study.
Focusing on the anti-inflammatory effect of 1-DNJ, several studies have been reported. In the gastric ulcer mouse model, 1-DNJ from soybean-derived B. subtilis had been proved to reduce the inflammatory cytokines TNF-α and IL-6 by measuring key molecules of the NF-κB pathway, as well as the level of NF-κB P65 (Piao et al., 2018). Also, 1-DNJ extracted from dried mulberry leaves had been reported to decrease the levels of TNF-α, IL-1β, and IL-6, alleviating macrovesicular steatosis (Liu et al., 2016). In another study, the mulberry leaf extract had been capable of dose-dependent reduction of the LPS-stimulated mRNA expressions of TNF-α, IL-1β, and IL-6 genes, which could be resulted from the presence of 1-DNJ in mulberry leaf extract (Park et al., 2013). Based on these reports, the synergistic inhibitory effect of LPS-induced IL-6 production by BVDSE compared with those by DSE and BSDSE could be caused by the 1-DNJ production by B. velezensis K26 during the fermentation of DSE.
Conclusion
Salicornia europaea L. (SE), a halophyte widely found in nature, has a biological properties including antioxidant, antidiabetic, and anti-inflammatory activities. As increasing the risk of high-salt intake, the desalination of SE has been increasingly interested and the desalted SE (DSE) has been accordingly developed with reporting its biological activities. In this study, we fermented the DSE using Korean fermented soybean paste (Doenjang)-derived B. velezensis K26 (BVDSE) which produces 1-deoxynojirimycin (1-DNJ) as one of wellknown α-glucosidase inhibitors. The BVDSE sample showed enhanced α-glucosidase inhibitory activity compared to that of DSE itself, which was proved to be resulted from the 1-DNJ production in the fermented BVDSE samples. In addition, the BVDSE sample was analyzed to reduce the lipid accumulation by inhibiting the differentiation from 3T3-L1 pre-adipocyte to adipocyte, suggesting the anti-obesity effect of BVDSE. Moreover, the BVDSE sample was shown to inhibit the IL-6 gene expression in LPS-induced RAW 264.7 cells, although it did not affect the TNF-α gene expression, representing the anti-inflammatory effect of BVDSE. These biological activities of BVDSE could be strongly attributed to the production of 1- DNJ in B. velezensis K26. Finally, the development of microbiologically- fermented DSE, in particular 1-DNJ-biosynthesizing B. velezensis, could be used as functional fermented food and biomaterial in diverse fields of bioindustry.







