Introduction
Mulberry (Morus alba L.) has been cultivated traditionally in Asian countries, such as Korea, China, and Japan (Fang et al., 2012). Mulberry leaves have long been used as feed for silkworms, and, recently, they have been reported to be effective for preventing and treating human adult diseases, such as diabetes, high blood pressure, and hyperlipidemia. Therefore, they are currently used as functional foods and medicines (Lee et al., 2003). Mulberry produces a variety of secondary metabolites, the major bioactive constituents of which include alkaloids, flavonoids, and terpenoids (Wang et al., 2018). 1-Deoxynojirimycin (DNJ), a polyhydroxylated alkaloid with anti-diabetic properties, is a bioactive compound found in mulberry leaves (Yagi et al., 1976). It was first synthesized using L-sorbofuranose or by reduction of nojirimycin; later, it was separated and refined directly from extracts of mulberry roots and silkworms (Kim et al., 2011). DNJ is a glucose analogue with an NH group in place of the oxygen atom of the pyranose ring (Kim et al., 2010). It is also known to be a strong human intestinal alpha glucosidase inhibitor (Yatsunami et al., 2008). DNJ binds to and fits into the active-site pocket of alpha glucosidase via hydrogen bonding (Yoshimizu et al., 2008), thereby preventing the uptake of postprandial blood glucose (Ju et al., 2015). For this reason, DNJ has been used for the prevention or treatment of type 2 diabetes, a non-insulin-dependent diabetes mellitus (Ji et al., 2016). The DNJ content of mulberry leaves varies according to the harvest period, genetic resource, growth environment, and so on (Yatsunami et al., 2008; Kim et al., 2013; De-Shuai et al., 2011). Although DNJ has various functional properties, the content of DNJ in mulberry leaves is very low, and the therapeutic or preventative use of DNJ from mulberry leaves thus requires a system that produces DNJ in high quantities.
Lactic acid bacteria (LAB) have been used for a long time in the production of fermented foods and beverages (Leroy et al., 2004). It has been reported that mulberry leaf powder extract (MLPE) fermented with Lactobacillus plantarum TO-2100 contains a higher level of DNJ than that in MLPE (Ryu and Kwon, 2012) and mulberry leaf extract (MLE) fermented with L. plantarum SDL 1413 contains a 1.2-fold higher DNJ content compared to that in MLE (Jeong et al., 2015). Microbial growth and the levels of DNJ produced during fermentation of MLE are affected by several intrinsic and extrinsic factors (Kwaw et al., 2017). Response surface methodology (RSM) is a useful statistical technique for identifying optimal fermentation conditions through a sequence of designed experiments. (Jiang et al., 2014). In this study, the major factors affecting MLE fermentation by lactic acid bacteria were determined, and RSM was used to identify the optimal values of these factors for obtaining enhanced levels of DNJ from MLE through fermentation.
Materials and Methods
The strains of Lactobacillus plantarum SG-053 and Pediococcus pentosaceus ON-35A used in this study were isolated from mustard leaf kimchi and onion, respectively (Choi et al., 2018). Seed cultures were obtained by growing the bacteria in Lactobacilli MRS broth (BD, Franklin Lakes, NJ, USA) at 37 °C for 24 h.
To determine the viable cell number of lactic acid bacteria in our samples, 1 mL of sample was diluted in 9 mL of saline (3M, Maplewood, MN, USA) by decimal dilution. Fifty microliters of the sample was spread on plate count agar with bromocresol purple (MB cell, Seoul, Korea), and colonies were counted after incubation at 37 °C for 24 h.
Mulberry leaves purchased from Jecheonyakcho (Jecheon, Korea) were extracted 10, 20, or 30 times distilled water at 100 °C for 2 h. To optimize the extraction time, mulberry leaves were extracted using an optimum volume of water at 100 °C for 1, 2, and 4 h. To optimize the roasting temperature, non-roasted mulberry leaves and mulberry leaves roasted at 100, 120, 140, 160, 180, and 200 °C for 5 min were extracted with an optimum volume of water, using a roasting temperature of 100 °C and an optimum extraction time. The mulberry leaf extract with the highest DNJ content was used to optimize the fermentation conditions according to the following procedure: first, the mulberry leaf extract was filtered with a cotton cloth and then centrifuged at 9,820 × g for 10 min (Beckman Coulter, Brea, CA, USA) to remove the mulberry leaves; next, the centrifuged filtrate was concentrated at 70 °C using a rotary evaporator (Heidolph, Nurnberg, Germany). Finally, the concentrated MLE was stored at 4 °C until further use.
Samples were centrifuged at 15,928 × g for 1 min (Labogene, Lillerød, Denmark), and 10 μL of the supernatant or a standard solution of DNJ (Sigma-Aldrich, St. Louis, MO, USA) was mixed with 10 μL of 0.4 M borate buffer (pH 8.5). Twenty microliters of 5 mM fluorenylmethyloxycarbonyl chloride (FMOC-Cl) (Sigma-Aldrich) in acetonitrile (J.T. Baker, Phillipsburg, NJ, USA) was added to the mixture and allowed to react at 20 °C for 20 min. Then, 10 μL of 0.1 M glycine (Sigma-Aldrich) was added to stop the reaction. The mixture was diluted with 950 μL of 0.1%(v/v) acetic acid (J.T. Baker) and filtered through a 0.22 μm PVDF syringe filter (Woongki Science, Seoul, Korea). Ten microliters of the filtrate was injected into a ZORBAX eclipse plus C18 column (4.6 × 250 mm, 5 μm; Agilent Technologies, Santa Clara, CA, USA) attached to an HPLC instrument (Dionex ultimate 3000 HPLC system, Thermo Fisher Scientific, Waltham, MA, USA) with a fluorescence detector (excitation 254 nm, emission 322 nm). The flow rate was 1.2 mL/min, and the mobile phase was a mixture of acetonitrile and 0.1%(v/v) acetic acid (40:60, v/v). The column oven temperature was maintained at 30 °C.
In order to check the effect of aeration on DNJ content, MLE was adjusted to 2.5 °Bx (Pocket refractometer, Tokyo, Japan) and pH 5.5, inoculated with 2.5%(v/v) of L. plantarum SG-053 seed culture, and fermented at 28 °C for 24 h at different agitation speeds using a shaking incubator (0, 50, 100, 150, and 200 rpm). To study the effect of inoculum concentration on DNJ content, MLE was inoculated with 1, 2, 3, 4, and 5%(v/v) of L. plantarum SG-053 seed culture and fermented at 28 °C for 24 h. To determine the effect of MLE concentration on viable cell count, MLE was adjusted to 1, 2, 3, 4, and 5 °Bx, inoculated with 4%(v/v) of L. plantarum SG- 053 seed culture, and fermented at 28 °C for 24 h. Finally, to determine the effect of inoculum concentration on DNJ content at different fermentation times, MLE was diluted to a concentration of 3°Bx; inoculated with 1, 2, 3, 4, and 5%(v/v) of L. plantarum SG-053 seed culture; and fermented at 28 °C for 24 h. The DNJ content was analyzed by sampling at 0, 12, 18, 24, and 36 h of fermentation.
A Plackett-Burman (PB) design was used to identify the three most important factors, among six (initial pH, fermentation temperature, fermentation time, inoculum concentration, MLE concentration, and agitation speed), that affect the DNJ content in fermented MLE. In this study, the pH was adjusted using edible Na2CO3 and citric acid (Hwami, Incheon, Korea). The PB design involved 12 runs, including triplicate at -1 and +1 levels.
Response surface methodology (RSM) was used for optimizing MLE-fermentation conditions using a central composite design. Independent variables of the initial pH (4, 5, 6, 7, 8), fermentation temperature (28, 31, 34, 37, 40°C), fermentation time (12, 18, 24, 30, 36 h), and levels (-2, -1, 0, 1, 2) were set according to the experimental design which involved 20 runs for triplicate experiments. Minitab 17.2.1 (Minitab Pty Ltd., Sydney, Australia) was used for response surface regression analysis.
The data are presented as the mean ± standard deviation (SD) of triplicate experiments. Statistical analyses were carried out using SPSS 23 (SPSS Inc., Chicago, IL, USA). Statistical significance between groups was determined using a paired t-test for repeated measures. Data with p values < 0.05, < 0.01, and < 0.001 were considered statistically significant. One-way ANOVA was used for comparison of group means, followed by Duncan’s multiple range test for determination of the significance of individual comparisons (p<0.05).
Results and Discussion
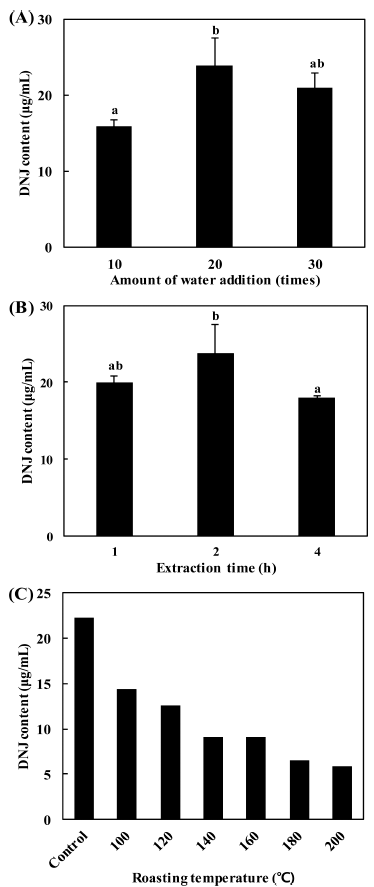
To determine the optimum conditions for mulberry leaf extraction, we examined the effects of water addition, extraction time, and roasting temperature on the DNJ content in MLE. As shown in Fig. 1, addition of distilled water 20 times weight of mulberry leaves (Fig. 1A), and extraction time of 2 h, and a roasting temperature of 100 °C (Fig. 1B) were the best conditions for the preparation of MLE. When tea is prepared using plant leaves, the leaves of some plants are roasted to enhance the organoleptic properties. When mulberry leaves were roasted at different temperatures, the DNJ content in MLE decreased as the roasting temperature increased (Fig. 1C). It has been reported that when silkworm powder was roasted at 121 °C, the DNJ content remained constant for only 15 min and then decreased (Yatsunami et al., 2011). Moreover, when silkworm powder was heated at 60, 100, and 150 °C for 30 min, the DNJ content in the silkworm powder heated at 150 °C was reduced to half (Ryu et al., 2013). This study demonstrated that roasting mulberry leaves reduced their DNJ content, and thereby, a roasting step was not applied to the extraction process in the present study.
The production of secondary metabolites by cultured microbes depends on fermentation conditions, such as the type and concentration of the culture medium, dissolved oxygen, pH, temperature, and agitation speed (Yoo et al., 2003). Among these, agitation speed greatly affects oxygen transfer rates during fermentation and the transfer of various nutrients in the medium available to microorganisms (Wecker and Onken, 1991). When we examined the effect of agitation speed on the production of DNJ, we found that the production of DNJ was highest without agitation (Fig. 2A). It has been reported that a stable dissolved oxygen concentration of 20% was required for the optimal production of DNJ by Streptomyces lavendulae (Kojima et al., 1995). This demonstrates that different microorganisms require different agitation speeds and dissolved oxygen concentrations for optimal DNJ production.
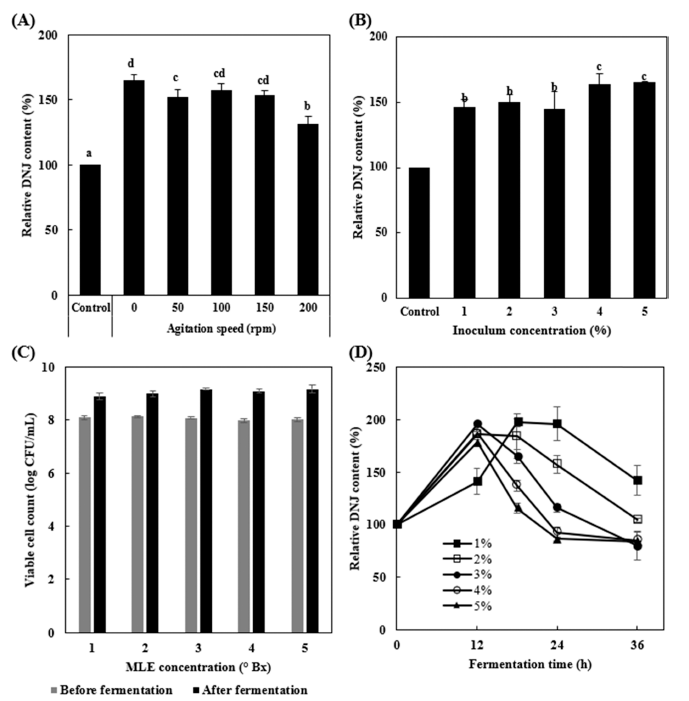
In the present study, the DNJ content increased as the inoculum concentration increased, as shown in Fig. 2B, and the optimum concentration of the inoculum was set to 4%(v/v) for further experiments. When the effect of MLE concentration on the growth of bacteria was examined, an MLE concentration of 3 °Bx showed the highest viable cell number (Fig. 2C).
The effect of fermentation time using different inoculum concentrations on the production of DNJ is shown in Fig. 2D. At 12 h of fermentation, the DNJ content was the highest at an inoculum concentration of over 2%(v/v); the DNJ content increased until 18 h of fermentation at an inoculum concentration of 1%(v/v). After 12 h of fermentation, the DNJ content decreased at an inoculum concentration of over 3%(v/v), while after 18 h of fermentation, the DNJ content at an inoculum concentration of 1%(v/v) was higher than that at an inoculum concentration of over 2%(v/v). Based on these results, we set the optimum inoculum concentration to 1%(v/v) because at this concentration, the DNJ content was highest after 18 h of fermentation and decreased slowly when compared with that at other inoculum concentrations.
To determine the optimum fermentation conditions for enhanced production of DNJ from MLE, we selected the following factors, with two levels being considered for each factor: initial pH, fermentation temperature, fermentation time, inoculum concentration, MLE concentration, and agitation speed. The Plackett-Burman design and the DNJ content after fermentation using this design are shown in Table 1. The highest DNJ content (36.63 μg/mL) was obtained using an initial pH of 5.5, a fermentation temperature of 28 °C, a 24 h fermentation time, a 2.5%(v/v) inoculum concentration, a 2.5 °Bx MLE concentration, and no agitation. Analysis of variance showed that the fermentation time, initial pH, and fermentation temperature were important variables affecting DNJ content (confidence interval [CI] greater than 95% (p<0.05).
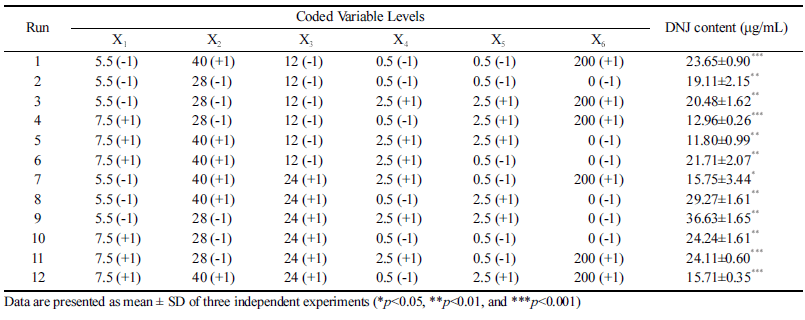
|
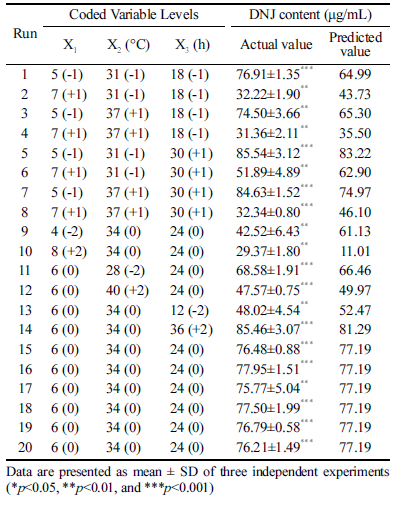
Based on this analysis, we selected the following factors for the optimization of fermentation conditions: initial pH, fermentation temperature, and fermentation time, with each factor having five levels. As shown in Table 2, it was confirmed that the DNJ content varied according to the variable level. The highest DNJ content (85.54 μg/mL) was obtained when the initial pH, fermentation temperature, and fermentation time were 5, 31 °C, and 30 h, respectively. The coefficients of the regression equation were calculated, and the following regression equation was obtained:
|
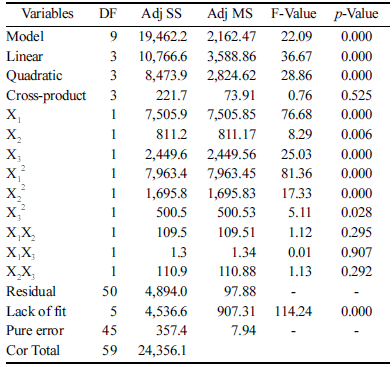
Where Y is DNJ content, X1 is the initial pH, X2 is the fermentation temperature, and X3 is the fermentation time. The analysis of variance of the reaction showed that the coefficient of determination (R2) was 0.7991. As shown in Table 3, a p-value of 0.000 indicated that the model was significant. In this study, X1, X2, X3, X12, X22, and X32 were significant model terms; especially, X1 had the greatest effect on DNJ content. Whereas X1X2, X1X3, and X2X3 were insignificant model terms. The actual value was close to the predicted value, as shown in Table 2, indicating that the regression model was acceptable.
|
Fig. 3 shows the effect of each variable on DNJ content as a three-dimensional plot (Fig. 3A-C) and contour lines (Fig. 3D-F). Based on the optimization studies, the optimized initial pH, fermentation temperature, and fermentation time were 5.5, 31.9°C, and 34 h, respectively, and the predicted content of DNJ was 87.21 μg/mL. This result showed that the DNJ content in MLE increased 3.59 times, from 23.85 to 85.54 μg/ mL, by fermentation using the optimal conditions determined through RSM.
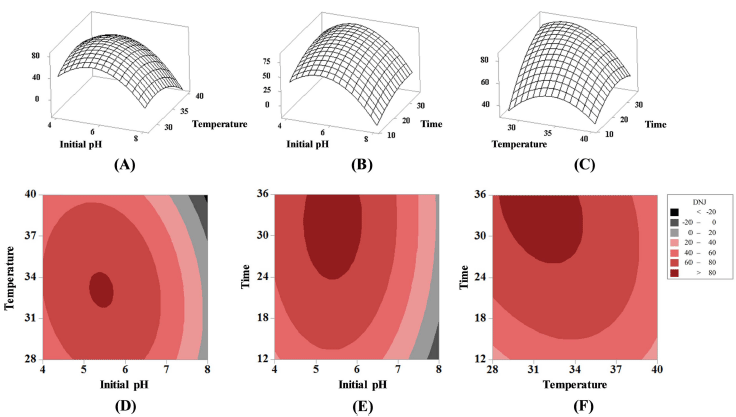
Jiang et al (2014) used RSM to optimize the conditions for enhancing DNJ production from mulberry leaf for enhanced production of DNJ from MLE through fermentation with Ganoderma lucidum. The optimal conditions, in this case, were a pH of 6.97, 0.81% of potassium nitrate, a 2% inoculum concentration. Under these conditions, the DNJ content increased 2.74 times. Wet et al. (2011) also used RSM to optimize the conditions for enhanced DNJ production through fermentation of mulberry leaves using Streptomyces lawendulae. The optimal conditions were identified as 11 days of fermentation time, 27°C of fermentation temperature, an initial pH of 7.5, and 8% of soluble starch; the expected value of DNJ content was 42.875 mg/L under these conditions.
This study is the first to report an enhancement of DNJ production from mulberry leaf extracts by lactic acid bacteria fermentation. It is expected that the results of this study will be useful for manufacturing functional foods that will be able to prevent or treat diabetes.







