Introduction
Recently, organic acids, phenolic compounds, and byproducts that are produced through the fermentation of plants have been recognized as beneficial for improving wellbeing and health (Song et al., 2011;Kim et al., 2016). The fermented plant can escalate the absorption of physiologically active substances into the body by the conversion of glycoside components that cannot be metabolized in the intestine (Bae et al., 2004). Fermentation also enhances flavor and taste, increases shelf life, removes toxic compounds, and increases the production of physiological and biologically active substances as polyphenolic components (Park et al., 2009).
Houttuynia cordata Thunb. is a perennial vegetable in the Saururaceae family commonly known as chameleon plant that is found mostly in Asia, including China, India, Korea, and Japan. This plant grows in shade and has a unique fishy smell in the leaves and stems. It is also called heartleaf owing to the shape of leaves. H. cordata is known to be effective in detoxification, facilitation of tissue regeneration, and treatment of a number of discomfort symptoms such as fever, swelling, and pus as a traditional remedy (Kumar et al., 2014). The plant has been used in medicine, tea, functional food products, cosmetics, and others (Kim, 2010). In 2003, a research group in China showed that H. cordata also can treat severe acute respiratory syndrome (SARS) (Kang & Koppula, 2014). H. cordata contains various physiologically active polyphenols such as kaempferol, quercitrin, quercetin, and alkaloids such as aristolactam, piperolactam, splendidine, and others, which have antioxidant (Cha et al., 2004), anti-hyperlipidemic (Chung et al., 1999), anti-inflammatory, anti-bacterial, antiviral, anti-allergic, and anaphylactic inhibitory activities (Kim et al., 2008;Kumar et al., 2014).
Studies on lactic acid bacteria fermented plants including Hijikia fusiforme Okamura (Song et al., 2011), root of Nelumbo nucifera and Platycodon grandiflorum (Lee et al., 2015a), fruit of Castanea crenata var. dulcis (Jun et al., 2014), and leaf of mulberry (Lee et al., 2015b) have been carried out. These plants fermented with lactic acid bacteria have been utilized as potential functional materials by increasing the total phenolic compounds and antioxidant activities after fermentation. Studies on the fermented H. cordata with the lactic acid bacteria were performed (Kim et al., 2016), but few fermentation studies have been conducted by L. brevis strain in plant materials (Hur et al., 2014).
Overweight and obesity are mainly caused by an abnormal accumulation of lipids in the body, and previous studies and clinical trials have focused on reducing accumulated body fat without side effects. In recent decades, extracts or phytochemicals from natural foods or plants has won attention as a remedy for obesity (Zhang et al., 2014). Single compounds from natural products such as epigallocatechin gallate (Bose, 2008), caffeic acid phenethyl ester (Shin et al., 2014), and capsaicin (Leung, 2014) have been reported as antiobesity botanicals. There have been preliminary reports of H. cordata, exhibiting an anti-obesity effect by inhibiting lipid accumulation in HepG2 cells, lipid absorption in mice, and improving non-alcoholic fatty liver as well as obesity (Miyata et al., 2010;Kang & Koppula, 2014).
To enhance these beneficial effects, this study investigated the effect of fermentation of H. cordata with Lactobacillus brevis B84 on the change in physicochemical components, antioxidant activity, and inhibitory effect on lipid accumulation in 3T3-L1 adipocytes and characterization of the polyphenol profile in Korean H. cordata tissue using high-performance liquid chromatography tandem mass spectrometry (HPLCMS/ MS).
Materials and Methods
Shade dried H. cordata Thunb, were purchased from Gyeongsangnam-do Jinju-si Farming/Agricultural Association Corporation. The sample was blended, and 1% of the sample was prepared in sterilized deionized water. L. brevis B84 was provided by the food microbiology lab at Gyeongsang National University (Kang et al., 2008). The bacterium was cultured in de Man, Rogosa, and Sharpe broth (MRS, Acumedia, Lansing, MI, USA) and incubated at 30°C foR20 h. The overnight grown culture (2%) was inoculated in the prepared sample with 1% glucose (Sigma-Aldrich Co., St. Louis, MO, USA) and incubated at 30°C for 48 h. After incubation, the fermented sample was filtered, freeze dried using a pilot lyophilizer (PVTFD50A, Ilshin Biobase Co. Ltd., Dongduchun, Korea). The extract was stored at -20°C until further use.
Total phenolic content was measured according to the Folin- Denis method with some modifications using gallic acid (Sigma-Aldrich Co.) as a standard (Gutfinger, 1981). The sample or gallic acid (1 mL) was mixed with 0.5 mL of Folin- Ciocalteu reagent. Then, 0.5 mL of 10% sodium carbonate solution was added and mixed. After incubation at room temperature (RT) for 1 h under dark, absorbance was measured at 760 nm. The total phenolic content of the sample was expressed as mg of gallic acid equivalent (GAE) using a calibration curve in 10-200 μg/mL gallic acid.
Quantification of simple phenolic compounds was conducted using an 1100 series HPLC system equipped with a G1322A degasser, G1312A pump, G1313A autosampler and G1316A oven (Agilent Technologies, Palo Alto, CA, USA). The chromatographic separation was carried out on a Shodex C18- 4D column (4.6×150 mm, 5 μm) set at 30°C was used. A 0.5% formic acid aqueous solution (v/v, solvent A) and methanol (solvent B) were used to establish the gradient elution as follows: 0-3 min at 10% B; 3-8 min linear gradient from 10% B to 20% B; 8-30 min linear gradient from 20% B to 90% B; and 30-35 min at 10% B for column equilibration. The mobile phase flow rate 0.9 mL/min and injection volume was 10 μL. Detector was PDA (Photo Diode Array, Agilent Technologies) at 280, 320 and 360 nm.
The color of fermented product was measured using a colorimeter (CR-301, Minolta Co., Osaka, Japan) for more than 20 times by placing 30 mL of each sample in a container of Φ 9 × 9 cm. The overall color difference (ΔE) of the sample was calculated with the L-value as lightness, a-value as redness, and b-value as yellowness. The L-, a-, and b-values of the standard color plate were 96.83, 0.29, and 0.32, respectively.
The pH of the sample before and after fermentation was repeatedly measured with 10 mL of 10% diluted filtrate (Whatman No. 6, Maidstone, UK) in distilled water using a pH meter (Model 720, Thermo Orion, Waltham, MA, USA). The acidity was determined by adding 9 mL of distilled water to 1 mL of the sample solution, and then measuring the amount of 0.1 N sodium hydroxide aqueous solutions consumed until the pH reaches 8.3.
Total sugar content was measured by mixing 1 mL of sample solution with 1 mL of 5% phenol solution and 5 mL of concentrated sulfuric acid, incubating at RT for 30 min, and measuring the absorbance at 470 nm using a spectrophotometer (Optizen 2120UV, Mecasys Co. Ltd., Daejeon, Korea) (Dubois et al., 1956). The reducing sugar content was determined by adding 3 mL of 3,5-dinitrosalicylic acid reagent (DNS) to 1 mL of the above sample solution according to Miller’s protocol (Miller, 1959). Then the mixture was heated in a boiling water bath for 10 min. The absorbance of the cooled sample was measured at 570 nm. The total sugar and reducing sugar content of samples was respectively calculated according to the standard calibration curve using glucose (Sigma-Aldrich Co.).
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity was determined by evaluating the electron donor activity based on Blois (1958). DPPH solution of 5 mg/100 mL methanol was mixed with the same volume of each sample and incubated at RT under dark for 10 min. The absorbance of remaining DPPH reagent at 517 nm was measured. To measuring ABTS (2,2'-azino-bis-3-ethylbenzothiazoline- 6-sulfonate) radical scavenging activity, 7 mM ABTS reagent dissolved in 2.4 mM potassium persulfate and incubated under dark for 12 to 16 h at refrigerated temperature. The mixture was diluted with water until the absorbance was reached to 1.5 at 415 nm. To 100 μL of the mixture, fermented product at each concentration was added and incubated for 5 min at RT. The absorbance was measured at 415 nm (Re et al., 1999). DPPH and ABTS radical scavenging activities were calculated as the inhibition percentage of the absorbance of fermented product to the control using deionized water.
Super oxide anion (O2 −) radical scavenging activity was evaluated by measuring the reduction of free radicals using nitro blue tetrazolium (NBT) as a substrate (Nishikimi et al., 1972). Fermented product at each concentration mixed with 10 μL of 0.1 M Tris-HCl buffer (pH 8.5) and 20 μL of 100 μM phenazine methosulfate (PMS) and incubated for 10 min at RT. The absorbance was measured at 560 nm (Co, So). Then 20 μL of 500 μM NBT and 20 μL of 500 μM β-nicotinamide adenine dinucleotide (NADH, reduced, disodium salt) were added to the mixture, and the absorbance was measured again at 560 nm (C, S). The radical scavenging activity was calculated by the following formula;
where “C” and “Co” are the absorbance of the control using deionized water with/without NBT and NADH, “S” and “So” are the absorbance of sample treatment with/without NBT and NADH.
Reducing power was measured by the FRAP method based on Benzie & Strain (1996). A substrate solution was prepared with 300 mM acetate buffer (pH 3.6) and 10 mM 2,4,6- tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and 20 mM ferric chloride (FeCl3·6H2O) in a 40 mM HCl mixture at a 10:1:1 (v/ v/v) ratio, and the solution was incubated at 37°C for 5 min. Forty microliters of fermented product, 40 μL of deionized water, and 100 μL of FRAP substrate solution were mixed and incubated at 37°C for 4 min. The absorbance was measured at 593 nm. Reducing power of the sample was calculated based on the standard calibration curve using iron (II) sulfate heptahydrate (FeSO4·7H2O) as an external standard.
3T3-L1 murine pre-adipocytes were purchased from ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO Laboratories, Grand Island, NY, USA) with 10% of fetal bovine serum (FBS; GIBCO) and penicillin/streptomycin at 37°C in a 5% CO2 incubator. To fully differentiate pre-adipocyte, 2.5×10 4 of 3T3-L1 cells were seeded in a 96-well plate and cultured foR2 days. After 100% confluency, cells were treated with a mixture of 5 μg/ mL of insulin, 115 μg/mL of 3-isobutyl-1-methylxanthine (IBMX), and 10 μM of dexamethasone (MDI) in 10% FBS/ DMEM foR2 days. The MDI/FBS/DMEM medium was changed to 10% FBS/DMEM containing 5 μg/mL of insulin foR2 days, and then replaced with 10% FBS/DMEM medium up to form lipid droplets in adipocytes. To quantify intracellular lipid content in fully differentiated adipocytes, an Oil Red O staining assay was performed. After washing with PBS solution three times, 10% formalin solution was used to fix the cells in the plate. After staining lipid droplets with Oil Red O solution (Sigma-Aldrich Co.) for 15 min, quantification was performed by determining the absorbance at 490 nm using a microplate reader (Varioskan, Thermo Electron Co., Waltham, MA, USA) after dissolving the staining solution in isopropyl alcohol. To determine the effect of the fermented products on adipocyte differentiation, resolved fermented products were treated in the medium at a designated concentration during differentiation. Inhibitory activation of the fermented products on adipocyte differentiation was calculated based on the control group (differentiated adipocyte treated vehicle) and shown as a % of control.
To determine the lipolysis ability of the samples, a glycerol release assay was conducted. Fully differentiated 3T3-L1 adipocytes were incubated with samples for 48 h, and the free glycerol concentration in the cultured medium was measured using a free glycerol determination kit (Sigma-Aldrich Co.). Lipolytic effect of the sample was calculated from the free glycerol content in the medium based on the standard curve using a glycerol standard solution (2.5 mg/mL).
Each experiment was repeated at least three times and the mean ± SD was calculated using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). The significant difference of each sample was performed by one-way ANOVA followed by a Duncan's multiple range tests and a Student-t test for unpaired comparisons. A value of p <0.05 was considered significant.
Results and Discussion
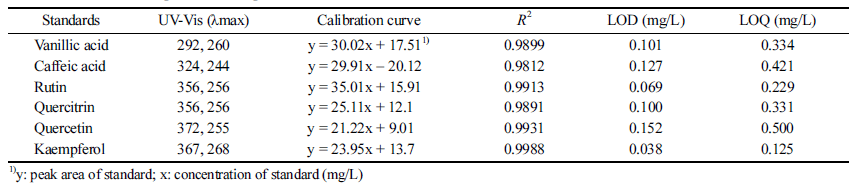
Quantification of those polyphenols was validated on the basis of a representative phenolic compound standard. Calibration curves were constructed via least-squares linear regression analyses of the ratio of the peak area of each representative compound to the peak area of the internal standard versus the added concentration of the respective compound. The regression equation was evaluated in the form of y = ax + b, where y and x represent the value of peak area and the concentration of each compound standard, respectively. Correlation coefficients (R2 ) were above 0.98. The performance limits were represented in terms of limit of detection (LOD) and limit of quantification (LOQ), with LODs below 0.152 mg/L and LOQs below 0.500 mg/L (Table 1).
|
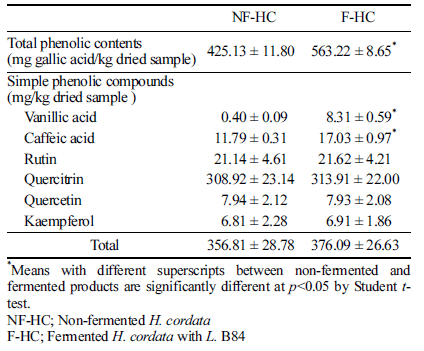
Total phenolic and six simple phenolic compounds were determined from the non-fermented/fermented H. cordata. Results were presented in Table 2. The six polyphenols characterized from non-fermented/fermented H. cordata were quantified using the peak areas of the LC chromatogram. But ρ-coumaric acid and ferulic acid were not quantified because trace amount of the compounds were detected. The total phenolic contents of the H. cordata significantly increased 1.3 fold after fermentation. Total amount of the quantified simple compounds of the fermented H. cordata was 376.09 mg/kg, which was also greater than that (356.81 mg/kg) of the nonfermented. The content of quercitrin was the highest (more than 80% of the total amounts), and there was no significant difference in the content of rutin, quercitrin, quercetin, and kaempferol after fermentation. However, contents of vanillic acid and caffeic acid were significantly increased in fermented product compared to the non-fermented.
|
Phenolic contents obtained by HPLC analysis were lower than those of the Folin-Ciocalteu assay. Studies related to these results have been reported; some phenolic compounds may be not detected by their HPLC condition or can be detected as trace amounts. And also it may be disturbed by other phenolic compounds and may not be detectable (Othman et al., 2009). Quantification of the total phenolic contents by gallic acid as standard in the Folin-Ciocalteu colorimetric method may also be an overestimated result (Schieber et al., 2001).
Increase of phenolic contents after fermentation indicated that the fermentation with Lactobacillus sp is closely related to the increase in botanical phenol and flavonoid content (Kim et al., 2016). Song et al. (2011) investigated the total phenol content of H. fusiforme extract fermented with L. brevis, there were significant differences between seven L. brevis strains, and they argued that the increase of total phenol content was due to the hydrolysis of H. fusiforme during the fermentation by Lactobacillus sp. Some phenolic compounds are known to exert positive effects including a strong radical scavenging activity (Guo & Wang, 2007). While vanillic acid and caffeic acid have been reported a lower correlation with antioxidant activity, total phenolic contents containing these compounds have been reported to show a significant difference from antioxidant activity (Chen et al., 2017). Therefore, total phenolic contents from fermented H. cordata may contribute to antioxidant activity.
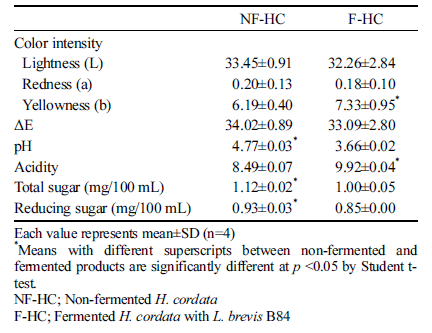
The physicochemical properties of fermented H. cordata with L. brevis B84 are presented in Table 3. There was no significant difference between the products before and after fermentation in the value of color other than yellowness. The pH and acidity significantly changed during fermentation; the pH decreased from 4.77 to 3.66 and acidity increased from 8.49 to 9.92 after fermentation. Genus Lactobacillus is more tolerant to acids than other lactic acid bacteria, and can grow well under pH 4.0-5.0 (Ha, 2000). L. brevis B84 was appropriate for this study by growing in the H. cordata extract with a decrease of pH after fermentation. Total sugar and reducing sugar contents of fermented H. cordata decreased significantly than before fermentation. These sugar contents of fermented H. cordata decreased by the conversion of sugars into organic acids such as lactic acid from using sugar as carbon source during fermentation. These results were consistent with reports by Song et al. (2011) on the reduction of reducing sugar by lactic acid fermentation in Hizikia fusiforme extracts. Kim et al. (1994) also showed that the reducing sugar content decreased as the acidity of fermented cabbage increased.
|
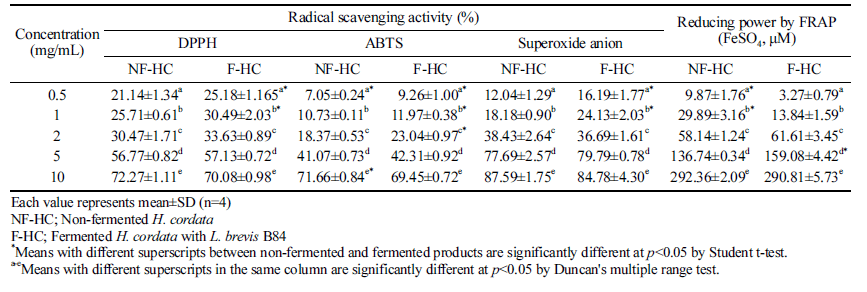
Antioxidant activities were compared from non-fermented and fermented products of H. cordata in 0.5-10 mg/mL, and the activity was significantly increased depending on the concentration of H. cordata as shown Table 4. DPPH radical scavenging activity was significantly increased after fermentation in the range of 0.5-1 mg/mL concentration. A significantly increase of ABTS radical scavenging activity after fermentation showed in the range of 0.5-2 mg/mL and 10 mg/mL. Superoxide anion radical scavenging activity showed similar tendency to their DPPH radical scavenging activity. On the other hand, reducing power by FRAP of the fermented H. cordata in 0.5-1 mg/mL was significantly higher in nonfermented product than fermented. However, reducing power of fermented H. cordata tended to increase at concentrations higher than 2 mg/mL, and the significant difference was observed at 5 mg/mL.
|
The increasing radical scavenging activity can be measured by the increasing reducing power of phenolic acid, flavonoids, and other phenolic substances as indicators of the antioxidant activities (Kang et al., 1995). Antioxidant properties derived from plants needs evaluation of their potential ROS scavenging activities in relation to polyphenol contents and components. Especially, the DPPH and ABTS radical scavenging activities are extensively utilized methods, because of their ease of use and a wide distribution of plant components (Suriyatem et al., 2017). DPPH accepts electrons or hydrogen radicals from hydrogen-donating antioxidants (Blois, 1958). On the other hand, ABTS assay is based on the inhibition of the formation of ABTS anion radical by one-electron oxidants (Tirzitis & Bartosz, 2010).
Fermented H. cordata with Leuconostoc mesenteroides 4395 and Lactobacillussakei 383 showed increasing DPPH radical scavenging activity depending on the concentration of the fermented product, otherwise, it has been reported that the fermented product showed a slight increase of superoxide dismutase (SOD)-like activity, and thus the result was different from DPPH radical scavenging activity (Kim et al., 2016). Lee et al. (2015b) reported that the radical scavenging activity of the mulberry leaf extract fermented with L. plantarum was increased. Sugar fraction of lotus root was fermented with L. delbrueckii and showed an 8% increase in radical scavenging activity. In addition, sugar fraction of Platycodon grandiflorum exhibited 177% increased radical scavenging activities when fermented with L. delbrueckii, 90% increased activities with L. brevis, and 77% with L. acidophilus (Lee et al., 2015a).
It is considered that the fermentation with lactic acid bacteria increased the radical scavenging activity while the increasing rate varies depending on the sample and inoculated bacteria strain (Wang et al., 2011). Fermentation of plantbased foods has positive influence on the total phenolic content and their antioxidant activity. On the other hand, after fermentation, the decrease of phenolic compounds is responsible for their antioxidant activities (Othman et al., 2009). It has also been reported that the difference in radical scavenging activity between non-fermented and fermented Agastache rugosa products with simple and mixed Lactobacillus strain was a little (Kim et al., 2015). Therefore, fermentation of H. cordata with L. brevis B84 has positive effect in terms of total phenolic content; however limited effect on the antioxidant activity.
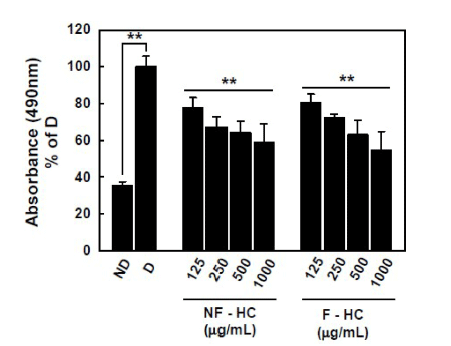
On the basis of the antioxidant activity of fermented H. cordata, we further evaluated the anti-obesity effect of fermented H. cordatain vitro. From the results of an Oil Red O staining assay, MDI and insulin treatment (D; differentiated group) in 3T3-L1 pre-adipocytes induced lipid accumulation and formation of lipid droplet by ~2.6 fold compared to a nondifferentiated group (ND). Co-incubation with non-fermented and fermented H. cordata showed reduced lipid accumulation compared with D group, but there was no difference in the reduced lipid accumulation ability according to the fermentation progress (Fig. 1).
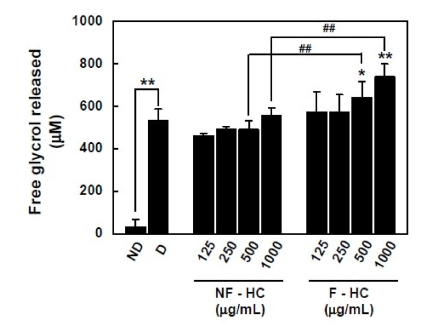
Strikingly, fermentation of H. cordata by L. brevis B84 induced lipolytic activity in 3T3-L1 adipocyte, these results are shown in Fig. 2. Non-fermented H. cordata did not show any ability for glycerol release activity, whereas fermented H. cordata significantly increased the amount of glycerol released from mature 3T3-L1 adipocyte, showing increased lipolysis by fermented H. cordata using L. brevis B84. Taken together, the present results show that fermentation using L. brevis B84 in H. cordata enhances their lipolysis while maintaining the reduction of lipid accumulation in 3T3-L1 adipocytes.
An increase in the concentration of the fermented green tea and H. cordata mixture gradually decreased on 3T3-L1 preadipocyte differentiation than non-fermented (Wang et al., 2018). It was demonstrated that feeding effect of water extract of H. cordata of 1-2% range improved triglyceride and total cholesterol in serum and liver tissue in mice fed a high-fat diet (Miyata at al., 2010). Kim et al suggested that polyphenolicrich extract of H. cordata has potent anti-atherogenic and antioxidant activities, and may be attributed to the quercetin (Kim et al., 2014). Also vanillic acid has the capacity to scavenge of superoxide anion and lipid radicals (Hamberg et al., 1974), and reported that significantly decrease the body weight in rats fed with high-fat diet (Vinothiya & Ashokkumar, 2017). The fatty acids cleaved from the mature lipids by lipoprotein lipase are imported into adipocytes and converted into triglyceride, and then lipogenesis of mature adipocytes is the main cause of obesity or hyperlipidemia.
These results suggested that the fermentation of H. cordata with Lactobacillus showed no loss of phenolic compounds, and its antioxidant activity and lipolytic effect are thought to be due to phenolic compounds.







