Introduction
Calcium is vital for building and maintaining strong bones and teeth, as well as for aiding muscle function, nerve transmission, and blood clotting (Soriano-Garcia et al., 1992; Jernigan & Resta, 2014; Capozzi et al., 2020). A long-term calcium deficiency leads to osteoporosis, a disease where bones become brittle and prone to fractures, and muscle cramps caused by impaired muscle function (Lanham-New, 2008). Calcium deficiency can affect the teeth and gums, leading to tooth decay, gum disease, and tooth loss (Abou Neel et al., 2016). Furthermore, hypocalcemia can impair the vision by causing cataracts and can affect the brain and nervous system by causing depression, memory loss, confusion, and seizures (Stern & Cardinali, 1994; Jafari et al., 2022).
The rate of inadequate calcium intake is high among Koreans. According to a study based on the 2020 Korea National Health and Nutrition Examination Survey (KDCA, 2021), the average calcium intake of adults in Korea was only 64.3% of the recommended amount, and 70% of the population do not meet the adequate intake level. In particular, calcium intake is low among specific groups such as women, young people aged 12 to 18 years, and elderly people aged 65 years or older.
Maintaining an adequate intake of calcium from food or supplements is important for optimal bodily functions and overall health. The calcium intake status in Korea can be improved by increasing availability and diversity of calciumfortified foods and beverages. In addition, the consumption of dairy products and other calcium-rich foods, such as leafy greens, nuts, seeds, beans, tofu, sardines, and salmon, should be promoted, and calcium supplements should be provided to high-risk groups, such as women, children, adolescents, and elderly people.
Volcanic seawater has been naturally filtered and purified through volcanic rock layers. It contains abundant minerals such as calcium (Ca), magnesium (Mg), iron (Fe), and other beneficial components that can contribute to health (Noh et al., 2010; Mohd Nani et al., 2016). Volcanic seawater is a natural source of calcium, and can be used as a calcium supplement (Song et al., 2020). Volcanic seawater has a high total dissolved solid content of over 2,000 mg/L, and it is classified as saline groundwater. Since the revision of the Drinking Water Management Act in 2010 National Law Information Center (NLIC, 2010), saline groundwater water has been utilized as a source of drinking water. However, no research has been conducted on the safety of calcium extracted from Jeju lava seawater (JLS). Therefore, genotoxicity tests must be conducted to establish the safety of food ingredients or functional materials derived from volcanic seawater.
Materials and Methods
JLS flowed through underground aquifers, naturally filtered by underlying volcanic rock, in eastern Jeju. The JLS at a depth of 150 m was uptaken and filtered to remove foreign substances. JLS mineral extracts and desalinated water were obtained using a reverse osmosis system (AG8040F-400 FR, Lenntech, Netherlands). Calcium from Jeju lava seawater (CJLS) was obtained by evaporating, washing, and drying the JLS mineral extracts using the difference in solubility.
This study was conducted in accordance with the Good Laboratory Practice regulations (Taegu Haany University, Korea) to investigate genotoxicity and determine whether CJLS directly damages DNA or chromosomes, leading to morphological changes or functional abnormalities. Genotoxicity tests included microbial reverse mutation tests, in vivo micronucleus tests using bone marrow cells, and chromosomal aberration tests (IACUC-2022-032-2).
For microbial reverse mutations, experiments were conducted in accordance with the Organization for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals (section 4 health effects test no. 471: bacterial reverse mutation test (OECD, 2020)). The mutagenic properties were determined using four histidine-requiring strains of Salmonella typhimurium (TA98, TA100, TA1535, and TA1537) and one tryptophan-requiring strain of Escherichia coli (WP2 uvrA). The strains used in this study were subjected to confirmation tests, such as amino acid requirements, UV sensitivity, rfa mutation, and R-factor maintenance, in accordance with Maron and Ames’ methods (Maron & Ames, 1983) to ensure that the genetic characteristics of the test strains were well preserved before proceeding with the experimentation.
The mutagenic positive controls used for inducing microbial reverse mutations in each strain included 2-nitrofluorene (2- NF, Sigma-Aldrich, Burlington, MA, USA), sodium azide (NaN3, Sigma-Aldrich, Burlington, MA, USA), 9-aminoacridine (9-AA, Sigma-Aldrich, Burlington, MA, USA), 4-nitroquinoline 1-oxide (4NQO, Sigma-Aldrich, Burlington, MA, USA), benzo(a)pyrene (BP, Sigma-Aldrich, Burlington, MA, USA), and 2-aminoanthracene (2-AA, Sigma-Aldrich, Burlington, MA, USA), and solvent controls included dimethyl sulfoxide (Sigma-Aldrich Burlington, MA, USA).
Test substances were prepared by dissolving 0.4 g of the test substance in 4 mL of solvent control and then diluted stepwise with solvent control, resulting in a final volume of 4 mL (highest concentration preparation). Testing was conducted through preincubation, with and without metabolic activation (+S9 mix and -S9 mix), with the metabolic activation system (S9 mix) purchased from MOLTOXTM (Molecular Toxicology Inc., Boone, NC, USA). Based on the results of the concentration determination test, the main test was conducted at five concentration levels (0, 61.7, 185, 556, 1670, and 5,000 μg/ plate) under metabolic activation and nonactivation conditions. The test substance was added to the top agar. After plating, precipitation and crystallization of the test substance were observed visually. Colonies grown on 90-mm-diameter plates were counted using a colony counter (SUNTEX model 570, Suntex instrument Co., New Taipei, Taiwan). The viable cell count was determined by diluting each strain 106 times, adding 0.1 mL of the diluted suspension onto a nutrient agar medium for culturing at 37 °C for 48 h, and counting the colonies.
The experiments for assessing the induction of chromosomal aberrations were conducted according to the OECD guidelines (2016). Chinese hamster ovary fibroblast (CHO-K1) cells were obtained from the Korean cell line bank (Seoul, Korea) and utilized to evaluate chromosomal aberration induction. Positive control substances, known to induce high sensitivity in CHO-K1 cells, were selected in accordance with the OECD guidelines. In the presence of metabolic activation (+S9 mix), cyclophosphamide (CPA, TOCRIS Bioscience, Bristol, UK) was used as positive control, and in the absence of metabolic activation (-S9 mix), mitomycin C (MMC, Sigma-Aldrich, Burlington, MA, USA) was used as positive control. Distilled water was used as the negative control. CPA was applied at a concentration of 10 μg/mL for 6 h, whereas MMC was applied at a concentration of 0.5 μg/mL for 6 h or 0.15 μg/mL for 12h.
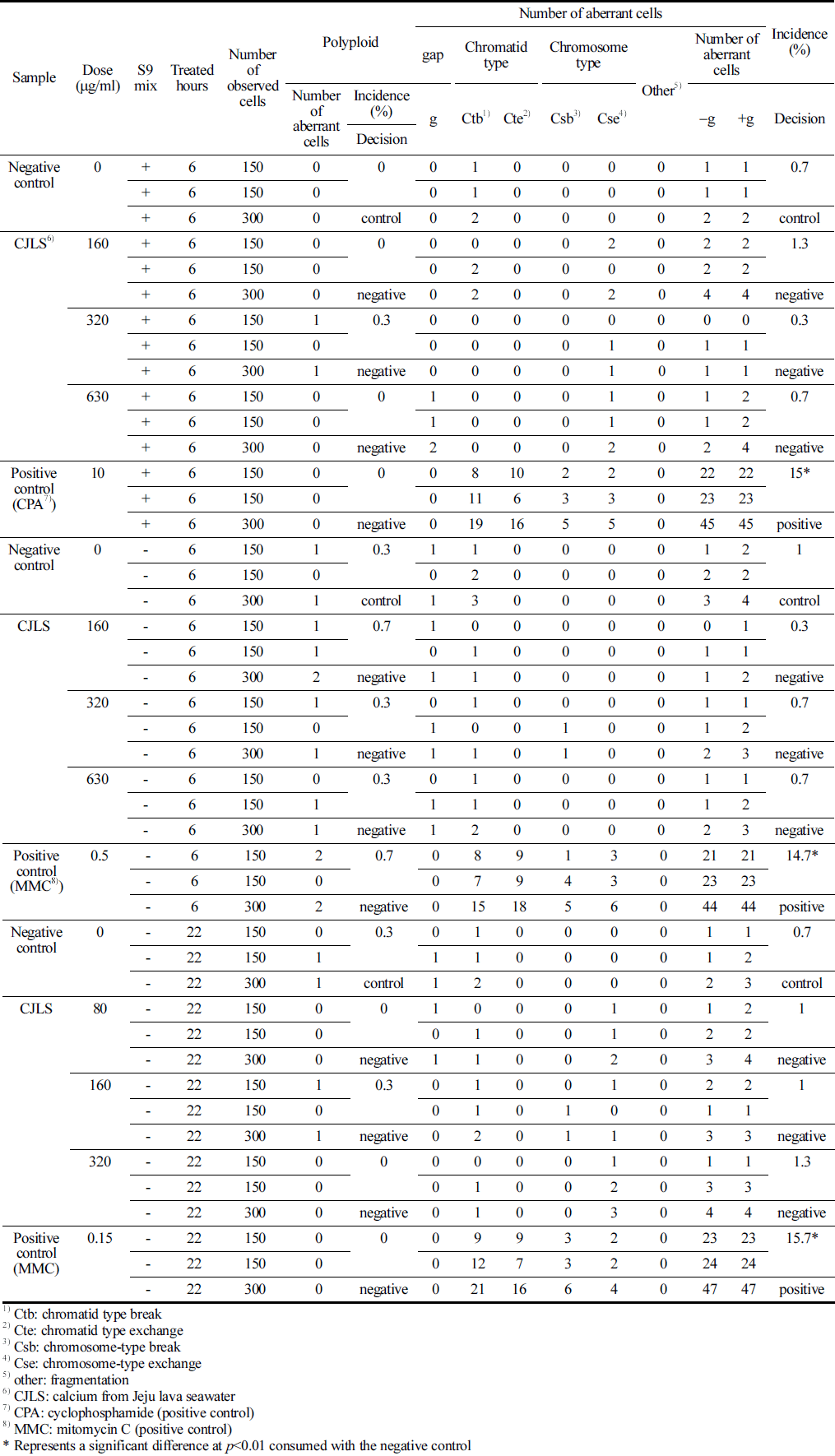
CHO-K1 cells were cultured using Eagle’s minimum essential medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum. The test substance was added with 2× 105 cells/5 mL in a 25-T culture flask and incubated for 3 days. Approximately 22 h after the initiation of test substance application for all flasks, 100 μL of colcemid (0.2 μg/mL) was added to each flask, and cells were collected after 2h incubation. After centrifugation and removing the supernatant, the cells were treated with a hypotonic solution of 75 mM KCl at 37 °C for 15 min. Cells were fixed thrice with a Carnoy’s fixative solution (methanol:acetic acid = 3:1), and 2 slides per flask were prepared from the chromosome samples. The slides were stained with a 3% Giemsa solution for 15 min and scored for chromosomal aberrations. Structural and numerical anomalies of chromosomes were analyzed by examining 300 metaphase cells per concentration (Table 1).
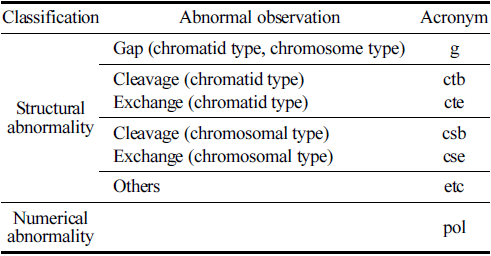
With regard to the observation criteria, a ‘Gap’ refers to an unstrained area spanning the major axis of the stained chromatin material, with a width roughly equal to the thickness of the chromatin material, clearly separating it. ‘Breakage’ is defined as a portion of the chromatin material detached from the major axis. ‘Exchange’ denotes the fusion of breaks in one or more chromosomes into two separate chromosomal parts or an exchange of chromosomal segments, which is clearly discernible. ‘Other’ signifies fragmentation, where numerous chromosomes display gaps, breakages, or exchanges, without exhibiting structural exchanges. Numerical anomalies were assessed by examining the polyploidy of CHO-K1 cells. The positive judgment criteria for chromosomal aberrations were defined on the basis of a dose-response relationship that is statistically significant at a significance level of p = 0.01, where cells with chromosomal aberrations significantly increase with dose, or at a significance level of p = 0.01, where a positive response is in one or more dose levels that is reproducible upon retest.
A total of 29 male ICR SPF mice aged 7 weeks were used in this study. The animals were purchased from Coatech (Seongman, Korea) and acclimated to the animal housing facility for 8 days before being utilized in the experiment. The animal facility maintained a temperature of 22.0±0.3 °C, relative humidity of 54.6±1.6%, a 12-h light-dark cycle (lights on at 08:00 and off at 20:00), lighting intensity ranging from 150 to 300 Lux, and noise level below 60 dB. The experimental animals were housed in polysulfone cages (280W× 420 D × 200 H) with no more than 5 mice per cage.
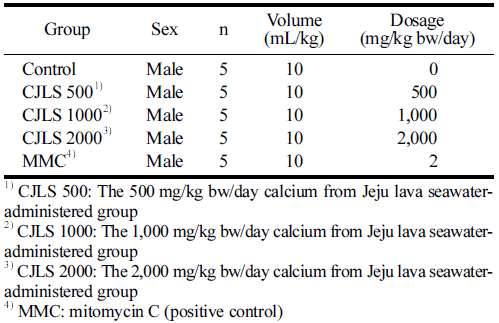
A certified irradiated solid diet for experimental animals (Teklad-certified irradiated global 18% protein rodent diet, Life Science, Indianapolis, IN, USA) and water was provided for ad libitum consumption. The CJLS was administered orally once a day, with a total of 2 administrations with a 24-h interval. The dose volume was 10 mL/kg, based on the body weight measured on the day of administration. In this study, the highest concentration was determined on the basis of the results of the dose-setting test and set at 2000 mg/kg bw/day. The study consisted of a solvent control group, 3 treatment groups (500, 1000, and 2000 mg/kg bw/day), and a positive control group (MMC 2m g/kg bw/day), forming a total of 5 groups (Table 2).
|
After CJLS administration, mice were sacrificed by cervical dislocation approximately 22 h later. Bone marrow cells were collected from the femur using fetal bovine serum centrifugation. Centrifugation was carried out at 1,000 rpm for 5 min; the supernatant was removed, and cells were resuspended in the serum. Cell suspension was smeared onto slide glasses, air dried at room temperature, and fixed with methanol for five min. Three bone marrow cell smear samples were prepared per hind limb. The bone marrow smears were stained with an acridine orange solution (40 μg/mL) for fluorescence staining, and micronuclei were observed in polychromatic erythrocytes (PCE). In stained samples, PCE that showed red fluorescence without a nucleus within the field of view were considered micronucleated, and normochromatic erythrocytes that showed no fluorescence and were only distinguished by shading were considered nonmicronucleated. Among 2,000 PCE, 250 polychromatic and micronucleated erythrocytes (MNE) were counted from one bone marrow smear sample. After counting, the frequency of MNE among PCE and the ratio of MNE to total erythrocytes were calculated.
The criteria for a positive result were as follows: a statistically significant increase in the dose-dependent occurrence of MNE, a positive response observed in one or more dose levels with reproducibility, and the occurrence of MNE exceeding the range of historical data of the solvent control group.
For the microbial reverse mutation test, the actual values, mean values, and standard deviations for the number of revertant colonies in each plate treated with the solvent control substance, positive control substance, and test substance were calculated. The fold-increase, which represents the ratio of the mean value of the test substance-treated group to the mean value of the solvent control group, was calculated to compare the mean values between the solvent control group and the test substance-treated group.
The results of chromosomal aberration judgment were analyzed using SPSS 19.0 (Chicago, IL, USA). Comparisons between the test group and the positive control group, as well as between the solvent control group and the test substancetreated group, were verified using chi-square tests and Fisher’s exact tests at a significance level of p = 0.01. If significance was observed, then the Cochran-Armitage trend test was conducted to confirm the dose-dependent relationship of chromosomal aberration frequency. In the micronucleus test, the significance test for the frequency of MNE and the ratio of MNE to total erythrocytes in the test substance-treated group compared with the solvent control group was performed using one-way ANOVA at a significance level of p = 0.05.
Results and Discussion
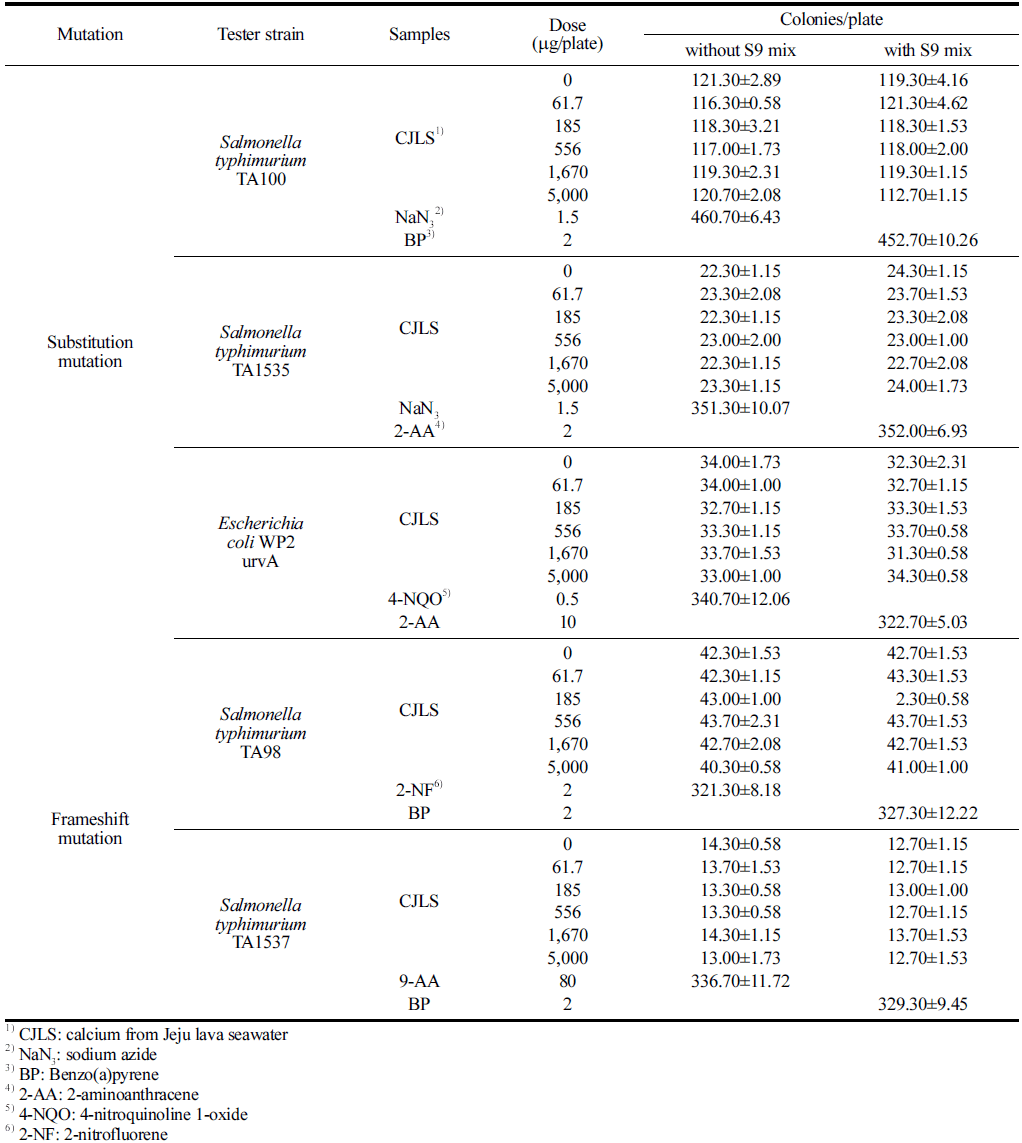
No significant growth inhibition was observed in the microbial reverse mutation assay using CJLS when compared with the negative control group for all five strains, regardless of the presence of metabolic activation (Table 3). The viable cell counts for the five strains used in this assay ranged from 1.15 to 1.24 × 109 /mL. Regardless of the presence of metabolic activation, the precipitation of the test substance was observed at 1,670 and 5,000 μg/plate for all strains, although a clear differentiation could be observed between the test substance precipitation and colonies.
|
No significant cytotoxicity, such as a decrease in revertant colony count and more than 50% fading of the background lawn, was observed in all strains at all concentrations of the test substance, regardless of the presence of metabolic activation. In addition, the number of revertant colonies in all concentrations of the test substance for all strains did not exhibit an increasing trend compared with the negative control group, regardless of the presence of metabolic activation. Therefore, CJLS does not induce reverse mutations in Salmonella typhimurium and Escherichia coli.
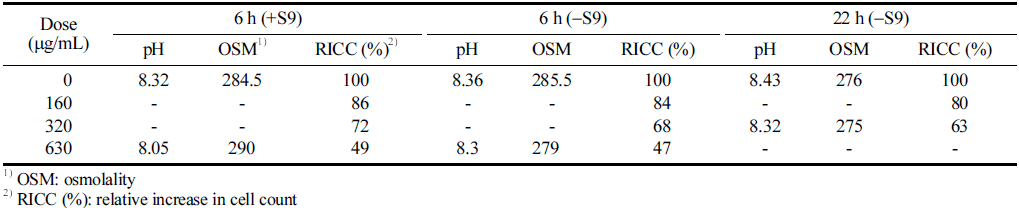
No significant changes in pH or osmotic pressure of the culture medium were observed during the treatment of the CJLS at the highest concentration for each group. The cell count was 8.5 × 105 cells/mL. The results of the concentration determination test indicated that the group treated for 6 h with the S9 mix exhibited a cell proliferation inhibition rate of 55±5% at a concentration of 0.63 μg/mL; the group treated for 6 h without the S9 mix showed the same rate at 0.63 mg, and the group treated for 22 h without the S9 mix showed a rate at 0.32m g/mL. Intermediate chromosome was also observed. Therefore, the chromosome abnormality test was conducted with the concentration showing the cell proliferation inhibition rate as the highest concentration (Table 4).
Based on the observations, the frequency of cells with structural chromosomal aberration at metabolic activation (+S9 mix) was 0.0%, 0.3%, 0.0%, and 0.0% at concentrations of 0, 160, 320, and 630 μg/mL, respectively, and 0.3%, 0.7%, 0.3%, and 0.3% at the same concentrations without metabolic activation (-S9 mix), respectively (Table 5).
No significant differences in the frequency of numerical and structural chromosomal aberrations were observed between the CJLS and the control group. However, for the positive control substances CPA and MMC treatments, significant differences in the frequency of structural chromosomal aberrations were observed between the positive control group and the negative control group (p<0.01). Therefore, the test substance, CJLS, does not induce numerical or structural chromosomal aberrations.
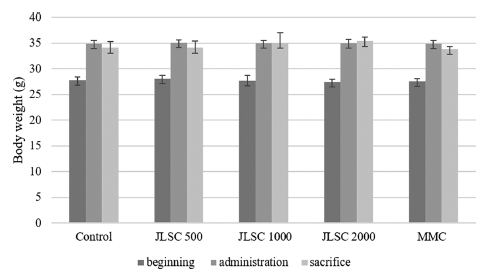
The result of the somatic cell test using male ICR mice showed that no deaths or abnormal symptoms were observed in any of the CJLS groups during the test period. Interval body weight changes caused by administration did not show significant differences compared with the control group for the 500, 1000, and 2000 mg/kg bw/day treatment groups, as well as the positive control group (Fig. 1).
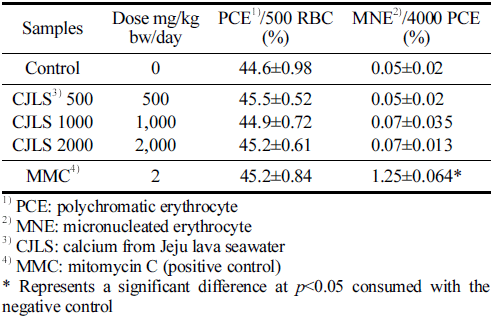
The results of the mouse bone marrow micronucleus test are presented in Table 6. The average ratio of PCE among 500 total erythrocytes per animal was not significantly different among the 500, 1000, and 2000 mg/kg bw/day administered groups; the positive control group; and the solvent control group. The frequency of MNE observed in 4000 PCE per animal was not significantly different in the 500, 1000, and 2000 mg/kg bw/day administered groups compared with the control group, and no dose-dependent response was observed. A significant difference was found in the positive control group compared with the negative control group (p<0.05). Therefore, the test substance, CJLS, does not induce micronuclei in murine hematopoietic cells.
|
This study demonstrated the absence of the genotoxicity of CJLS, a natural extract with potential therapeutic benefits. Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer (Choudhuri et al., 2021). Therefore, genotoxicity testing is an indispensable component in the safety assessment, which aims to prevent certain substances from affecting human health. Considering that no single test is capable of detecting all relevant genotoxic end points, a combination of in vivo and in vitro testing techniques for genotoxicity is recommended (Madle et al., 1994). In this study, three standard tests for genotoxicity, namely, bacterial reverse mutation assay, chromosomal aberration assay, and mouse bone marrow micronucleus test were performed. The results revealed that CJLS did not induce reverse mutations, chromosomal aberrations, or micronuclei in any of the test systems, indicating that CJLS is not genotoxic under the experimental conditions. This finding provides valuable information on the further development and application of CJLS as a safe and effective natural product.
CJLS as a functional food can provide various healthpromoting effects without causing any adverse effects on the genetic material of the consumers. Functional foods have potentially positive effect on health beyond basic nutrition (Granado-Lorencio & Hernández-Alvarez, 2016) and help reducing the risk of chronic diseases, enhance the immune system, and improve the quality of life (Alkhatib et al., 2017; Ashaolu, 2020). However, some functional foods may also contain substances having genotoxic potential, which can damage the DNA and cause mutations or cancer (Choudhuri et al., 2021). Therefore, it is important to ensure the safety and efficacy of functional foods before marketing or consumption. CJLS, as a nongenotoxic functional food, can enhance the health and well-being of the consumers. CJLS contains bioactive compounds that have antioxidant and anti-inflammatory effects (Noh et al., 2010; Lee et al., 2019). The minerals in CJLS likely mitigate hepatotoxicity by enhancing the antioxidative and anti-inflammatory capabilities of the liver (Lee et al., 2019). These compounds can modulate the metabolic pathways and gene expression involved in various physiological functions and disease prevention such skin pigmentation-related disorders (Song et al., 2020). Therefore, CJLS can be considered as a safe and effective functional food for improving the health status and quality of life of the consumers.
Calcium plays a crucial role in human physiological functions, and natural calcium supplements aid human health. JLS may serve as a natural calcium supplement and thus, toxicity studies have been conducted using Ca extracted from JLS.
The present study revealed that CJLS exhibits no genotoxicity. Testing of the substance through various assays such as the bacterial reverse mutation assay, the chromosomal aberration assay, and the mammalian micronucleus test, indicated no mutagenic potential. CJLS did not induce mutagenicity in Salmonella typhimurium TA98, TA100, TA1535, TA1537, and Escherichia coli WP2uvrA when tested with the metabolic activation of the S9 mixture. CJLS did not induce significant chromosomal aberration in CHL cells in the presence or absence of S9 activation. Furthermore, the oral administration of CJLS did not significantly increase the number of micronucleated PCE or the mean ratio of polychromatic to total erythrocytes. Therefore, CJLS can be considered as a reliable and safe functional food ingredient.