Introduction
Among all cancer incidences in Korea, gastric cancer accounts for 17.1% of men and 9.2% of women (Korea Central Cancer Registry, 2018). Since the risk of gastric cancer increases in proportion to the grade and range of atrophic gastritis, a process of chronic inflammation of the gastric mucosa (Sipponen et al., 1985), diagnosis of atrophic gastritis is essential for early screening of gastric cancer. A continuous inflammatory reaction causes atrophic gastritis, and gastric glandular structures are destroyed, causing thinning of the mucous membrane and a lightening of the mucous membrane (Rugge et al., 2002). Gastritis development is complexly related to age, smoking, alcohol, Helicobacter bacteria, food factors (high-salt diet, burned food, etc.), and genetic factors. Since there is no specific treatment for gastritis, daily life management should be necessary (Ko et al., 2018).
Chronic inflammation in the stomach is caused by the action of inflammatory mediators such as interleukin (IL)-6, nitric oxide (NO), and tumor necrosis factor-alpha (TNF-α). NO is manufactured by immune cells, such as macrophages, and plays an essential role in pathological processes (Moncada et al., 1991). Franco & Talamini (2009) reported that NO production was greatly increased due to H. pylori, a representative factor in inducing gastritis. TNF-α has been associated with viral defense, and TNF-α is considered one of the most important cytokines involved in the induction of NO (Fonseca et al., 2003), which provides the second signal for inducting inducible nitric oxide synthase (iNOS) (Liew et al., 1990). IL- 6 also plays a vital role in the host's response to tissue damage and infection (Pawłowska-Kamieniak et al., 2021). It has been reported that the expression of soluble IL-6 receptor (sIL-6R) is increased in chronic gastritis patients with H. pylori infection, which is thought to be related to IL-6 trans-signaling in gastric epithelial cells (Chen et al., 2018). Azadegandehkordi et al. (2015) reported that enhanced induction of IL-6 may be involved in the pathogenesis of gastritis.
Damage to the gastric mucosa causes inflammation, and histamine, which is secreted during inflammation, causes serious mucosal damage. To protect the gastric mucosa, it may be helpful to prevent the release of histamine secreted from the stomach since the typical mucosal damage treatment is used as type 2 histamine receptor antagonists, which inhibit histamine receptor activity (Chen et al., 2006). Additionally, there are gastric acid neutralizer as a significant treatment of gastrointestinal disorders, gastric mucosa protective agents, and proton pump inhibitors that inhibit gastric acid secretion for the treating gastrointestinal diseases. However, these drugs have a gastric acid inhibitory effect and have a relaxing effect on the smooth muscles of the gastrointestinal tract, which can lead to severe abdominal bloating.
Cabbage (Brassica oleracea var. capitata) is widely contained in cruciferous vegetables, and glucosinolate is converted into stable substances, such as isothiocyanate thiocyanates and nitriles etc. during cooking. Major isothiocyanate has been reported as a major component in the anticancer effect (Soundararajan & Kim, 2018), and sulforaphane among isothiocyanate acts as anticancer by inducing translocation of Nrf2 into the nucleus (Holloway et al., 2016). There have been reports that it attenuates the degree of gastritis caused by Helicobacter. Cabbage also contains a large amount of vitamin U (S-methylmethionine, SMM), which inhibits gastric juice secretion and promotes cell regeneration in ulcer tissue. It provides gastrointestinal protection mainly through the mucus production (Watanabe et al., 1996;Ichikawa et al., 2009). Ichikawa et al. (2009) reported that the inhibition of gastric surface mucous cell function with famotidine was alleviated by SMM and Watanabe et al. (1996) also reported a protective effect against mucosal damage.
It may be possible to treat gastric inflammatory diseases by suppressing the production of inflammatory mediators, such as NO and IL-6, and regulating histamine production. Many studies have been reported on the cytoprotective role of cabbage extract or sulforaphane against experimentally induced gastritis (Fahey et al., 2002;Yanaka et al., 2009;Yamada et al., 2014;Chang et al., 2015). However, research on whether cabbage directly regulates IL-6, NO, and histamine production and expression are insufficient. Therefore, this study investigates the effect of the degree of gastrointestinal mucosal damage and plasma histamine secretion in an HClinduced gastric mucosa injury animal model.
Materials and Methods
The cabbage used in this experiment was bought from Jeju Island as a “Dae Bak Na” in 2019. The edible part of the cabbage was finely cut and dried at 45 °C for 54 h in a hot air dryer (KUKJE ENG CO., Goyang, Korea). After drying, the prepared cabbage powder 25 g was extracted at 40 °C for two hours. After concentrating on a rotary vacuum concentrator (EYELA, Rikakiki Co., Tokyo, Japan), the stock solution was stored at -20 °C and used as a raw material for the test substance.
Sulforaphane and SMM in cabbage extract were quantified using HPLC on an Agilent HPLC system (Agilent Technology, Santa Clara, CA, USA) using an RStech OptimaPak C18 column (5 μm; 4.6×150 mm; RS tech Cooperation, Daejeon, Korea). The mobile phase consisted of acetonitril and deionized water (30:70), introduced at a flow rate of 0.8 mL/min. The detection wavelength was set to 202 nm. The cabbage extract was standardized to contain 0.625-20 mg/mL of sulforaphane. SMM was analyzed by Hong & Kim (2005) method on an HPLC system equipped with a fluorescence detector. A lyophilized powder sample was used and analyzed using a Zorbox Eclipse AAA-C18 column (3.5 μm; 4.6×150 mm; Agilent Technology, Santa Clara, CA, USA). The mobile phase of solvent A (40 mM NaH2PO4, pH 7.6-7.8) and solvent B (acetonitrile:methanol:water = 45:45:10) was used at a flow rate of 2.0 mL/min. Optimum excitation and emission wavelengths were 340 nm and 450 nm, respectively. Concentrations including 50, 100, 200, 400, and 800 μg/mL SMM standard solutions were prepared for analysis. In the obtained cabbage extract, the concentration of sulforaphane was 5.19 mg/L, and vitamin U was 469.28 ug/L under this experimental condition.
Raw 264.7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM: Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, penicillin 100 U/mL, streptomycin 100 ug/ml. Raw 264.7 cells were seeded at 4×104 /well in a 96-well plate containing DMEM and incubated for 24 h. The cells were then incubated at different cabbage extract concentrations (sulforaphane conc. at 39-2,500 μg/L) in DMEM for 48 h. The cell viability was determined using the EZ-cytox (DOGEN bio, Seoul, Korea) at 450 nm using a microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
NO level was measured in the cultured medium using the Griess reaction (Weissman & Gross, 2001) of NO2, which is a stable NO oxide. Briefly, 0.1-mL macrophage culture supernatant was added to a 96-well plate. The Griess reagent (Promega, WI, USA) was added, and the samples were incubated for ten minutes at room temperature, and the same amount of Griess reagent was added to react for ten minutes. The absorbance of the sample was measured at 540-nm using a spectrophotometer (Spectracount, Packard, Warrenville, Il, USA). For IL-6 and TNF-α, raw 264.7 cells were seeded at 4 × 104 /well in a 96-well plate and incubated overnight. The cells were treated with a medium containing the cabbage extract for seven hours, and 500 ng/mL lipopolysaccharide (LPS) was added. The supernatant was collected at 48 h after cell seeding, IL-6 and TNF-α concentrations in the supernatant were measured according to the manufacturer's instructions using each cytokine ELISA kit (BD Bioscience, Franklin Lakes, NJ, USA).
ICR (8-weeks-old, female) were bought from Saeronbio Ltd. (Uiwang, Korea) and maintained in a specific pathogenfree room at Kyonggi University. Animal experiments were conducted according to the guide- lines of the Ethics Committee for the use of Experimental Animals at Kyonggi University (2018-002). The animals were supplied with water and a pellet diet ad libitum. After 24 h of fasting, 15 mice were randomly divided into five groups: control group, normal saline; alcohol group (nega- tive control); E1 group, alcohol+ E1 (5.75 mg/kg); E2 group, alcohol+ E2 (11.5 mg/ kg); and E3 group, alcohol+ E3 (23 mg/kg). Samples were administered orally using distilled water as a vehicle. Thirty minutes after sample administration, 350 μL 40% EtOH in 150 mM HCl was orally administered to induce gastritis. After an hour of HCl/EtOH administration, animals were sacrificed by cervical dislocation, and their stomachs were excised. Excised stomach tissue was fixed with 3% formalin, and the degree of gastric damage was observed.
One hour after sample administration, 350 μL 40% EtOH in 150-mM HCl solution was administered through oral gavage to induce gastric lesions. The mice were euthanized by cervical dislocation one-hour post-HCL-EtOH administration. The stomachs were quickly removed, then washed with sterile saline. The stomachs were stretched on a flat plate and photographed to analyze the gastric lesions. The gastric lesion area was measured using Image analysis (Optimas 5.2, Optima, Brookfield, WI, USA). The sampling tissue was digitized to a computer database using light microscope (Zeiss, Thornwood, NY, USA) and then magnified 4 ×. The degree of hemorrhagic abrasion was obtained through the following equation.
In the animal test, the inflammatory state of the stomach was evaluated using histopathological scoring, which is scored from 0−5 points. For histopathological scoring, hematoxylin & eosin stains were used by Gim et al. (2015) method. Tissue sections were deparaffinized with xylene and rehydrated with ethyl alcohol. The slides were rinsed using distilled water and immersed in hematoxylin for 10 min. The slides were then kept under running water in a glass jar for tenminutes and treated with 1% HCl and 1% ammonia water. The slides were added to the eosin solution for 5-10 min. After drying, the slides were dehydrated in graded ethyl alcohol (70%, 95%, and 100%) and cleared. Finally, the slides were pictured using a light microscope (BX50, Olympus, Tokyo, Japan) and analyzed by ImageJ, a computer-based program.
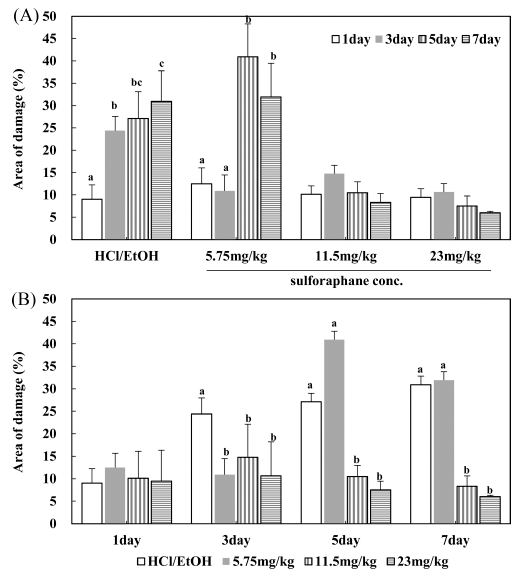
The score of 0 was the absence of inflammatory reactions in the mucous membrane or submucosa, and the score of five was the presence of neutrophils and macrophages infiltrating the mucous membrane, submucosa, muscle layers, extensive necrosis, swelling, erosion, and ulcers in all parts of the gastric epithelium. And the erosion of the gastric mucosa was confirmed among the samples.
All data values obtained are indicated as mean ± standard deviation. The significant difference between the two groups was analyzed using Student t-test. The significant difference among groups was statistically analyzed using one-way analysis of variance followed by post-hoc Tukey’s test (p< 0.05). All calculations were conducted in SPSS v22.0 (SPSS Inc., Chicago, IL, USA).
Results
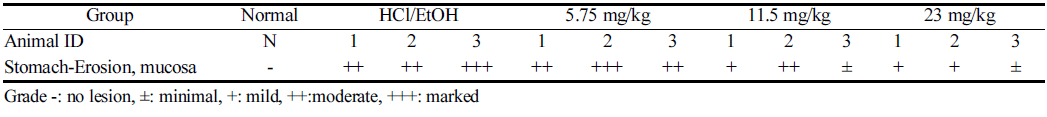
To establish a range of noncytotoxic concentrations for cabbage extract, RAW 264.7 cells were treated using different concentrations based on sulforaphane 78 μg/L, 156 μg/L, 312 μg/L, 625 μg/L, 1,250 μg/L, and 2,500 μg/L for 48 h. As indicated in Fig. 1, the concentration 39-1,250 μg/L for cabbage extract did not affect cell viability compared to control.

As indicated in Fig. 2(A), LPS treatment significantly induced NO levels in the control group (p<0.05). However, the cabbage extract inhibited NO release in Raw 264.7 macrophages stimulated by LPS. Sulforaphane at a concentration of 39-312 μg/L significantly reduced NO release compared to the LPS treated cell in a dose-dependent manner.
The results of the IL-6 level produced in each culture supernatant after obtaining the macrophage culture media are indicated in Fig 2(B). The LPS exposure group showed a significant increase in IL-6 compared to the normal group (p<0.05). However, it was discovered that the level of IL-6 induced by LPS tended to reduce in a dependent manner, and cabbage extract at a concentration of 312 μg/L sulforaphane significantly reduced the level of IL-6 induced by LPS (p<0.05).
For TNF-α levels produced in each culture, supernatants are indicated in Fig. 2(C). The LPS exposure group had a significantly higher level than the normal group (p<0.05). Meanwhile, TNF-α level induced by LPS indicated a significant attenuation at concentrations of 156 μg/L and 312 μg/L sulforaphane (p<0.05).
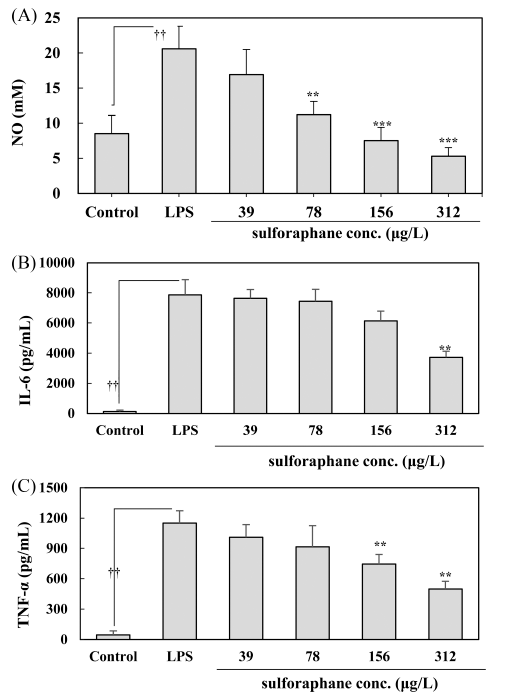
In the vehicle group with distilled water, mucosal damage was undetected. As the treatment period increased, gastritis was induced in the HCl/EtOH group; hemorrhagic bleeding marks were observed in the gastric tissue, resulting in severe hemorrhagic mucosal damage in the entire stomach. Mice treated with HCl/EtOH showed a significant increase in gastric damage (33.37±6.88%) compared to vehicle-treated control mice on the seventh day. In contrast, mice treated with cabbage extract reduced damage area (%) induced HCl/EtOH (Fig. 3(A)). In the results of the concentration of 11.5 mg/kg and 23 mg/kg, the degree of damage to the gastric mucosa was ameliorated by approximately 75-80% depending on the concentration.
And it was confirmed that the degree of damage to the gastric mucosa was inhibited according to the treatment period (Fig. 3(B)). And it was found that the damaged area reduced to a percentage within 8.5% when the cabbage extract was treated from 5-7 d. Especially, treatment with 11.5 mg/kg and 23 mg/kg sulforaphane significantly recovered to a normal condition (p<0.05). Cabbage extract dose-dependently inhibited gastric mucosal damage, with the greatest reduction of gastric mucosal damage observed at 23 mg/kg sulforaphane.
The data of microscopic examination of stomachs are indicated in Fig. 4 and Table 1. In the normal group, no specific findings were found in the gastric mucosa. As a result of the speculum, erosion of mucous membranes was observed in the HCl/EtOH group, the test substance administration group (sulforaphane at 5.75 mg/kg, 11.5 mg/kg, and 23 mg/ kg), compared to the normal group. The degree of mucosal erosion was the most severe in the HCl/EtOH group, and it was observed that the degree of erosion was reduced in the order of 5.75 mg/kg, 11.5 mg/kg, and 23 mg/kg among the test substance administration groups. The gastroprotective effect of gastric mucosa was observed when the concentration of 23 mg/kg was treated on the bases of sulforaphane.
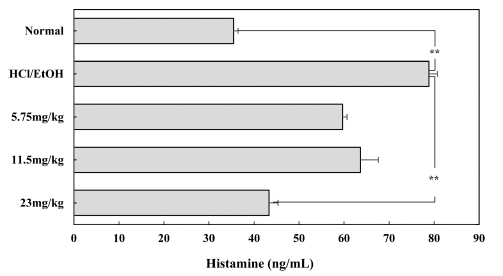
Histamine, which is released by gastrin and acetylcholine irritation of the gastric mucosa, causes more acid secretion and damage to the mucous membrane during gastric irritation or inflammation, which aggravates symptoms. When the plasma histamine concentration was measured using an animal model, the histamine level was significantly higher in gastritisinduced animal models (p<0.05). However, histamine secretion was dose-dependently reduced in HCl-EtOH-treated mice that received sulforaphane at a dose of 5.75-23 mg/kg.
Discussion
Macrophages are distributed in all tissues in the animal body and are immune cells responsible for the innate immune response and play an essential role in the human immune system. Macrophages activated by external stimuli induce an inflammatory reaction through inflammatory mediator secretions, resulting in asthma, bronchitis, arthritis, multiple sclerosis, arteriosclerosis, and stroke (Albina & Reichner, 1995). LPS, one of the endotoxins, increases pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β in macrophages, NO, prostaglandin E2 (PGE2), and other inflammatory mediators. In the inflammatory state, cyclooxygenase-2 (COX-2), an enzyme that mediates the biosynthesis of different prostaglandins (PGs), and NO synthase (NOS), an enzyme that manufactures NO, are induced to generate excessive amounts of PGE2 and NO, and different diseases and carcinogenesis are promoted (Lee & Lim 2008). Particularly, NOS and COX are important mediators for regulating the inflammatory response (Lawrence et al., 2002), and NO has a pro-inflammatory effect as a secondary signal transducer in cells (Anggard, 1994). This study shows that the cabbage extract using macrophages has anti-inflammatory activity, suggesting that cabbage extract can be considered a mechanism for inhibiting inflammatory diseases.
The previous study (Socała et al., 2017) showed that high doses of sulforaphane has toxicity, including marked sedation at sulforaphane concentrations of 150-300 mg/kg, impaired motor coordination at 200-300 mg/kg, and the decrease of skeletal muscle strength at 250-300 mg/kg. However, since sulforaphane has been considered as a low-toxic dietary phytochemical through several studies (Brown et al., 2015;Wu et al., 2016), in this study, the cytotoxic range was confirmed and the effects of cytokines were investigated. The pro-inflammatory cytokine is an important indicator of inflammation. TNF-α and IL-6 are representative substances of pro-inflammatory cytokine and activates the final stage of differentiation of B-cells into plasma cells and increase inflammatory lesions (Kim et al., 2011). In this experiment, LPS treatment significantly increased IL-6 in macrophages, inhibited the IL-6 level of 25% at 156 μg/L concentration based on sulforaphane, and IL-6 at 50% level at 312 μg/L concentration. Since IL-6 has stimulates iNOS expression to promote NO secretion (Yang et al., 2007), this study suggests that IL-6 and NO levels increased by LPS are sequentially suppressed by cabbage extract. Jia et al. (2007) reported that ROS plays a significant role in directly or indirectly mediating gastric tissue damage by stimulating inflammatory signaling pathways. In line with these findings, these results were demonstrated to inhibit the production of TNF-α and NO in the LPS-stimulated RAW264.7 macrophages after treatment using cabbage extract.
Ethanol dissolves gastric wall mucus and can impair the gastric mucosa rapidly. This means the gastric mucosa can be easily damaged by hydrochloric acid (Oates & Hakkinen, 1988;Bento et al., 2018). Additionally, HCl/EtOH produces a mucosal region with a necrotizing area by disrupting the protective mucus-bicarbonate barrier (Júnior et al., 2014;Ribeiro et al., 2016;Bento et al., 2018). In this experiment, at doses of 11.5 mg/kg and 23 mg/kg, cabbage extract significantly prevented HCl/EtOH-induced gastric damage lesion by 70% and 81%, respectively, compared to the HCl/ EtOH-induced model. These results propose that cabbage extract has gastroprotective effects on HCl/EtOH-induced gastric mucosal injury.
There are many chemical mediators related to chronic inflammation. The released chemical mediators, included vasoactive amines, such as histamine and serotonin, bradykinin, eicosanoids, such as thromoxanes, leukotrienes, and prostaglandins (Abdulkhaleq et al., 2018). Histamine is a mediator involving in allergic reactions and also performs homeostatic functions such as intestinal bowel control (Branco et al., 2018;Hirasawa, 2019). The combining of histamine to eosinophil H4R upregulated expression of macrophage 1 antigen (Mac1) and ICAM-1 adhesion molecules and promotes actin filament rearrangement (Ling et al al., 2004;Barnard et al., 2008). Rearrangement of actin filaments induces to the gathering of eosinophils to the site of inflammation. Histamine amplifies the inflammatory reactions on the inflamed site and prolongs the inflammatory response, eventually inducing chronic inflammatory status.
Histamine also regulates the inflammatory process by modulating on other cell populations (Marone et al., 2001;Triggiani et al., 2001). For the acute-phase inflammatory response, IL-8, IL-1beta, TNF-alpha IL-6, and IL-12 are the most remarkable secretory cytokines (Kany et al., 2019). The generation of animal toxicity is mainly attributable to the secretion of IL-1beta, IL-6, TNF-alpha due to exposure to LPS of pathogens (Shivachandra et al., 2011).
Histamine causes more acid secretion and damage to the mucous membrane during gastric inflammation, which is associated with TNF-α, IL-6, and IL-10 levels (Klausz et al., 2003). The immunomodulatory reaction of histamine proceeds by each receptor, and cytokine production is managed through H1 and H4 receptors, and gastric acid secretion is regulated through H2 receptors (Fitzsimons et al., 2001). Cytokines are essential in the inflammatory process and it acts as an early factor in the progress of disease. Recently, in the case of H4 antagonists, it has been reported that the anti-inflammatory effect appears in serious chronic inflammatory diseases such as diabetic nephropathy (Pini et al., 2018), arthritis (Cowden et al., 2014), and colitis (Varga et al., 2005). Thus, it has been recognized that histamine plays more roles in different diseases than had been predicted. In the oxygen mucosa of the mammalian stomach, histamine mainly accumulates in enterochromaffin-like (ECL) cells and mucosal mast cells (Andersson et al., 1998). In animal models, more than 80% of the oxygenated mucosal histamine is found in ECL cells. Histamine is a crucial element in regulating gastric acid secretion, and histamine receptor antagonists play a role in inhibiting the production of gastric acid by acting on histamine 2 receptors in gastric parietal cells (Chen et al., 2006). In this study, the treatment of cabbage extracts suppressed inflammatory cytokine release and plasma histamine. This means that cabbage has anti-inflammatory effects and inhibits histamine secretion.
In summary, severe mucosal damage was observed in the gastritis-induced control group. The degree of gastric mucosal damage was reduced depending on cabbage extract treatment. When the plasma histamine concentration was measured using an animal model, the histamine concentration was increased in the gastritis-induced animal model, and histamine secretion was reduced in the 23 mg/kg-treated cabbage extract group.
There are many scientific reports regarding the gastroprotective effects of cabbage extract. Although it has beneficial effect on gastric health due to its antioxidant and antiinflammatory effects, few studies relate histamine, which is one of the gastric mucosal defense systems. This study demonstrated the gastroprotective effect of cabbage extract, and this gastroprotective effect is mediated by a mechanism of action involving inhibiting histamine and chronic inflammation. This study showed that cabbage extract could help inhibit gastrointestinal disorders by improving the protective barrier.