Introduction
Westernized dietary habits have increased the intake of highcalorie foods and thus increased the incidence of various adult diseases, including obesity, hypertension, and diabetes (Jung et al., 2011). As interest in functional foods to reduce adult diseases has increased, the functional roles of dietary fiber have been in the limelight. Studies have revealed the antimutagenic effects of insoluble dietary fiber derived from greenish-yellow vegetables and soybeans (Lee et al., 1995) Chinese cabbage (Liu et al., 2012), and oak (Chai et al., 2003).
Opuntia ficus-indica, OFI, (var. saboten), belonging to the fan-cactus family, grows mainly in the southern parts of Korea. The stems and fruit of OFI contain 30% water-soluble dietary fiber, which can effectively treat constipation, improve digestive function, and enhance appetite (Set et al., 1999). SDF has a high water-holding capacity and easily forms a gel, which allows food to stay in the stomach longer and delays digestion. Due to these properties, dietary intake of SDF is recommended to increase tolerance to diabetes (Torsdottir et al., 1911). In addition, SDF adsorbs cholesterol and bile acids in the intestines, thus promoting their excretion into the feces, altering lipoprotein metabolism and lowering serum cholesterol levels (Kay and Truswell, 1977).
In recent years berries have received attention as functional raw materials and have been incorporated into various beverages (Lee et al., 2000;Bae et al., 2002;Son and Lee, 2004). Recent studies have confirmed the nutritional composition (Seo et al., 2016), antioxidant activity (Jeong et al., 2016), and bioactivity of OFI (Lee et al., 2016). However, there has been little research on OFI stem extracts and methods to optimize their yield and quality.
Therefore, in this study, the process of extracting watersoluble dietary fiber and solids from OFI stems was optimized, and the functionalities of the optimum extract were investigated.
Materials and Methods
OFI stem powder was harvested in Jeju, Korea, in 2016 and spray drier was used to powderize. Ethanol was purchased from Duksan Pure Chemical Co. (Ansan, Korea), and a total dietary fiber assay kit (K-TDFR 10/14, Megazyme, Bray, Ireland) was used for dietary fiber analysis. As reagents for measuring antioxidant activity, ascorbic acid, 1,1-diphenyl-2- picrylhydrazyl (DPPH), 2,2'-azino-bis (3-ethylbenzthiazoline- 6-sulfonic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2,2'-Azobis (2-amidino-propane) was purchased from Wako Pure Chemical Industries (Osaka, Japan).
The extraction process was varied by extraction time, extraction temperature, and solvent pH. Ten grams of OFI stem powder were mixed with 1 L of solvent. Then, the mixture was homogenized for 10 s with a centrifugal homogenizer (Ultra-Turrax T-25, Staufen, Germany) for total 30 min. The extracted solution was centrifuged (3000×g) in a centrifugal separator (VS-6000CFN, Bucheon, Korea) at 4 º C for 30 min, and the supernatant was filtered and dried at 60 º C for 24 h.
The amount of soluble dietary fiber (SDF) was analyzed with a total dietary fiber assay kit (K-TDFR 10/14, Megazyme, Bray, Ireland). The SDF content was determined by the following equation, and each experiment was repeated three times.
where M1R and M2R are two determinations of the residual weight of the sample (mg), P is the protein weight (mg) in residue M1R, A is the ash weight in residue M2R (mg), B is the blank content (mg), and M1 and M2 are two determinations of the original weight of the sample (mg).
Design expert 8P (Stat-Easy Co., Minneapolis, MN, USA) was used to design the experiments and optimize the extraction conditions (Han et al., 2003). Fifteen experimental points, 5 experimental points for lack of fit, and three replication points were set based on simplex centroid design, and the constraint values were set as the SDF and solid contents. Through a preliminary experiment, the range of each extraction condition was determined to be 50-70 º C, pH 7-8, and 3-7 h as a result of the preliminary experiment. After optimization was performed by numerical analysis with the canonical model, the suitable component ratios were selected. Numerical optimization sets goals for each response to the number of models based on the canonic model and predicts desirability.
The OFI stem extract solution was mixed with 50 mL of 70% ethanol and centrifuged at 1,000 rpm at 50 º C for 30 min. The sample was filtered with a 0.45 μm PVDF membrane filter (Whatman, Buckinghamshire, UK) and analyzed by a modified version of the method of Jeong et al. (2016). The mobile phase was 0.1% formic acid (A) and distilled water with 0.1% formic acid (B). The gradient for HPLC analysis was changed linearly as follows: 85% A/15% B, 82% A/18% B, 80% A/20% B, 80% A/20% B, 60% A/40% B, 10% A/90% B, 10% A/90% B, 95% A/5% B and 95% A/5% B. The flow rate was set at 1.0 mL/min at 23℃. The detection wavelength was set at 320 nm for phenolics in OFI.
The DPPH radical scavenging activity of the OFI stem powder extract was determined by the method of Cho et al. (2011). A DPPH radical solution (100 μM) was prepared in 80% (v/v) methanol. Then, 50 μL of the sample solution was added to 2.95 mL of the DPPH radical solution and reacted at 23 º C for 30 min. The amount of DPPH radical was measured at 517 nm. The DPPH radical scavenging activity was expressed as mg vitamin C equivalents (VCE)/100 g dry weight.
The antimicrobial activity of the OFI stem powder extract against Escherichia coli and Bacillus cereus was determined based on optical density. These bacteria were cultured in LB medium and BHI medium, respectively, at 37 º C for 24 h with different amounts of stem extract (10, 20, 30, and 50%). The optical density was measured on a spectrophotometer (Beckman Coulter DU 730, USA).
Results and Discussion
Ten grams of OFI stem powder were mixed with 1 L of ethanol (0, 20, 40, 60 or 80%), and SDF and solids were extracted at 60 º C for 90 min (Fig. 1A). The SDF content was the highest value (22.27%) when water alone was used as a solvent. As the percentage of ethanol increased to 20 and 40%, the extracted SDF content was lowered to 15.07 and 15.17%, respectively. Further increases in ethanol content to 60 and 80% resulted in SDF yields of 10.10 and 11.39%, respectively, demonstrating that higher ethanol content caused a lower yield of SDF. Similarly, hot water extraction was reported to produce the highest yield of SDF from dropwort, while higher concentrations of ethanol hampered the extraction (Won et al., 2015).

In the solid content extraction (Fig. 1B), there was no significant difference between water and 20% ethanol (4.83 and 4.94 g). However, further increases in ethanol concentration to 40 and 80% reduced the solid content yield to 3.82 and 1.46 g, respectively. Thus, unlike the SDF yield, the solid content yield did not change when up to 20% ethanol was used as the extraction solvent. Lee et al. (2005) reported that water extraction from cactus was more effective than any ethanol concentration, and we obtained similar overall results in this extraction.
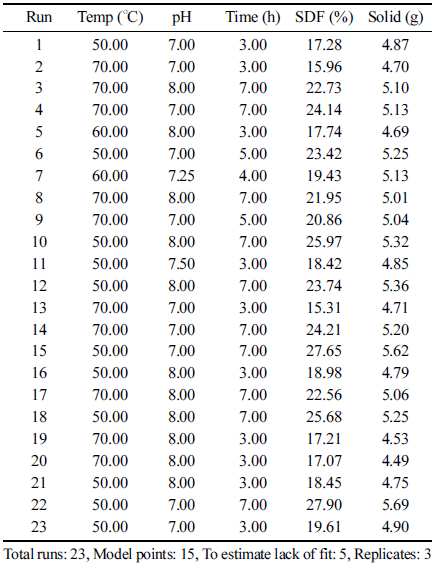
Among the various extraction conditions that could affect the yield of OFI stem powder extract, temperature, pH, and time were set as independent variables. The proximate extracted SDF and solid contents from the stems under the actual extraction conditions are shown in Table 1. The SDF and solid contents ranged between 15.31-27.90% and 4.49- 5.69 g, respectively, depending on the temperature, pH, and time. In general, a lower temperature (50 º C) and a longer extraction time (7 h) resulted in higher SDF and solid contents. When the extraction temperature and time were the same, the extraction yields of SDF and solids were higher at lower pHs.
|
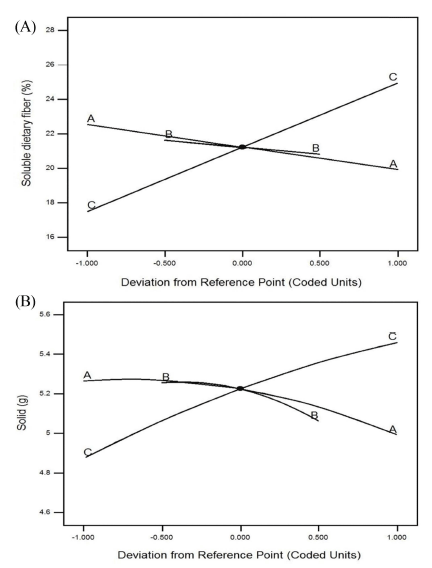
The probability values and predicted model equations of the selected non-linear models were calculated. The 2FI model was selected for SDF, while the quadratic model was selected for solids. The probability values for the SDF and solid contents were 0.0007 and 0.0496, respectively. Due to the low probability of each model, both models were used to describe the effects of the extraction conditions on the SDF and solid contents and were applied in the optimization process. A trace plot was used to confirm the relationship between each extraction condition and the yield of extracted materials (Fig. 2). The slope of the trace plot revealed the impact of each extraction condition on the response (Han et al., 2003). As shown in Fig. 2, the SDF and solid contents tended to decrease with increasing extraction temperature (A-A) and extraction solvent pH (B-B). However, as the extraction time (C-C) increased, the yields of SDF and solids increased. The slope of the extraction temperature curve (A-A) was flatter than that of the extraction time curve (C-C), indicating that the extraction time was more influential than the extraction temperature. The extraction yields of both SDF and solids were greater in acidic conditions, similar to the results of Aoe et al. (1993).
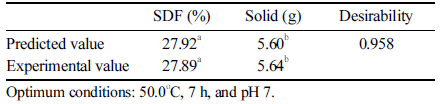
In the prediction of with the optimum numerical values for the extraction conditions, the SDF yield was 27.92% and the solid yield was 5.60 g (Table 2). The optimum extraction conditions were 50 º C, pH 7, and 7 h, and the desirability was 0.958. The higher was the desirability value, the better were the objective factors, and the desired results were in accordance with the goal of optimization. In a reproducibility test under the optimum extraction conditions, an SDF content of 27.89% and a solid content of 5.64 g were obtained; thus, there was no significant difference between the predicted and real experimental values.
|
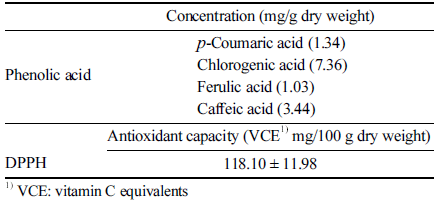
The phenolic acid composition of the stem extract obtained under the optimum extraction conditions was analyzed, and four phenolic acids were detected, as shown in Table 3. Chlorogenic acid was the most abundant phenolic substance among the standard products tested (7.36 mg/g DW), followed by 3.44 mg/g DW caffeic acid, 1.34 mg/g DW p-coumaric acid, and 1.03 mg/g DW ferulic acid. According to Guevara et al. (2010), 11 species of Opuntia cacti were detected, and ferulic acid was detected in all cultivars, whereas gallic acid and ρ-coumaric acid were not found. The amounts of phenolic compounds in our extract were much higher than those reported by other researchers. Jeong et al. (2016) found that chlorogenic acid was 2.24 μg/g DW, ferulic acid was 1.57 μg/g DW, and ρ-coumaric acid was 54.24 μg/g DW. The differences in the amounts of phenolic compounds detected in this study may have been due to optimization of the extraction process.
|
The antioxidative activity of the OFI stem extract was evaluated based on its DPPH radical scavenging activity and the results are shown in Table 3. The DPPH radical scavenging activity was 118.10 mg.
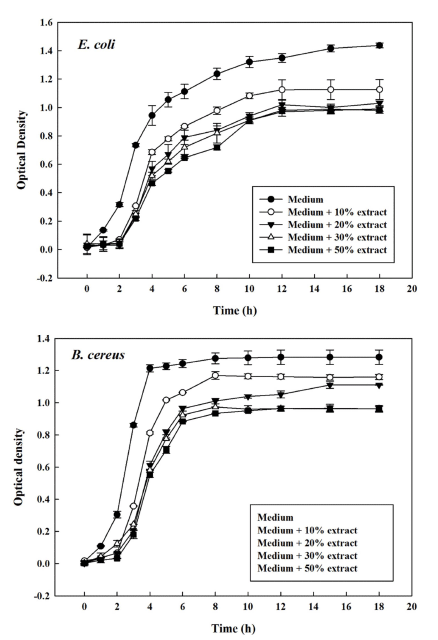
The antimicrobial activity of the OFI stem powder extract was determined based on optical density when bacteria were cultured with different concentrations of the extract (Fig. 3). In the case of E. coli, growth inhibition was observed in the medium without extract, and the final optical density was 1.437. When the extract was added to the medium, microbial growth was reduced by 34%, and the final optical density was 0.98 with 50% extract. In the case of B. cereus, the time to reach the final optical density was shorter than that of E. coli, and the final optical density was 1.283 without OFI stem extract. Significant differences in optical density were found when up to 30% extract was used. The final optical density was 0.962 for both 30% and 50% extracts, indicating that the antimicrobial activity was well inhibited in the medium containing 30% extract.