Introduction
In previous papers, the preparation (Park, 2005) of crystalline β-1, 4-mannotriose from poonac using β-mannanase from Trichoderma harzianum and some properties (Park, 2006) of purified mannanase have been reported. Crystalline β-1,4- mannobiose has been prepared from poonac using the enzyme system and yeast fermentation (Park, 2005). Using the β- mannanase of Streptomyces, β-1,4-mannooligosaccharides (Kusakabe et al., 1983) were prepared for the application of functional manno-oligosaccharides. It was further reported (Park, 2005) that direct hydrolysis of the poonac by mannanase is easier and more economic for preparing mannooligosaccharides. Mannooligosaccharides have been prepared from partial acid and enzymatic hydrolysates of plain mannans. However, the traditional preparation methods are not suitable to prepare a substantial quantity of mannooligosaccharides because of the low yields and complex process. As there are few natural sources of mannan, β-1,4-mannooligosaccharides was prepared (Kusakabe et al., 1983) using the β-mannanase of Steptomyces. In above paper, I concluded that direct hydrolysis of the poonac by mannanase is easier and more economic for preparing mannooligosaccharides.
I previously reported the characteristic features of α- galactosidase from Penicillium purpurogenum (Park et al., 1991) and properties of purified mannanase (Park et al., 1987). There are many reports dealing with β-mannanase from various microorganisms (Tipson & Horton, 1976; Akino et al., 1998; Dekker, 1983), but only three kinds of enzymes (from konjac tubers (Shimahara et al., 1975), Tyromyces sp. (Shimizu & Ishihara, 1983), and Streptomyces sp. (Takahashi et al., 1984) have been studied in terms of the specificity of the enzyme to glucomannan. In this lab, the hemicellulose hydrolysates from microbial hemicellulase are separated to prepare a Modified MRS medium for growth of intestinal bacteria. In this study, Xylogone sphaerospora hemicellulase (β-mannanase, α-galactoside) is used and Candida guillieromondii was selected because of fermentation to monosaccharides. The objective of this paper is to apply the specific characteristics of the enzyme and carry out the preparation of MGM from konjac glucomannan using a combined process. The combined process consists of hydrolyzing the konjac using the crude enzyme and eliminating monosaccharides from the resulting hydrolysate with yeast.
Materials and Methods
Konjac glucomannan was a gift from Tsuruta Shokuhin Kôgyô Co., Ltd. (Gunma-ken, Japan). The ratio of mannose to glucomannan glucose was 1.0:0.6. The mannooligosaccharides were prepared using the method described in a previous paper (Kusakabe et al., 1983). Another reagents used in this experiment were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The mannanase from Xylogone sphaerospora (KCCM 60478) was prepared using submerged culturing, as described in a previous paper (Lee & Park, 2008a; Lee & Park, 2008b). The resulting culture filtrate was dialyzed at 4 ° C overnight against a 4-fold volume of distilled water and was then used as the mannanase solution in the hydrolysis of the konjac.
The assay mixture, containing 129.4 mg of copra mannan (Park et al., 1987) (equivalent to 100 mg of polymannose), 4.0 mL of McIlvaine buffer solution (pH 6.0), and 5 mL of water was put into an L-shaped tube. The tube was then preincubated at 60 ° C for 10 min on a monod shaker with agitation at the speed of 60 oscillations per min. One mL of the enzyme solution was added to the mixture, which was then incubated for 30 min at the same temperature. The reducing power produced by the enzyme reaction was determined as mannose using the Somogyi method (Somogyi, 1945). One unit of the enzyme activity was defined as the amount of liberated reducing sugar equivalent to 1 μm of mannose per min under the above conditions.
Reducing sugar was determined using the Somogyi method (Somogyi, 1945). Total sugar content in the enzymatic hydrolsate was determined by the same method, after hydrolyzing oligosaccharides by 4% H2SO4 at 100 ° C for 2 h.
Nutrients (consisting of 0.2 g peptone, 0.3 g yeast extract, 0.1 g each of potassium phosphate (monobasic) and magnesium sulfate, and 0.2 g of calcium carbonate were added to 100 mL of the supernatant liquid of enzymatic hydrolysate of konjac. The resulting medium was placed in a 500 mL shake flask and sterilized at 120 ° C for 5 min in an autoclave. The seed culture of the yeast was inoculated into the medium and cultivated at 30 ° C on a reciprocal shaker. At certain time intervals, a small amount of culture broth was removed from the flask, followed by the removal of yeast cells by centrifugation. After insoluble materials were removed from the resultant hydrolysates at 24 and 48 h by centrifugation, each 4 μL of the supernatant liquid was subjected to TLC.
The konjac (20 g), which contained 10.2 g of total sugar (with 6.4 g of mannan), was hydrolyzed with 200 mL of the enzyme solution at pH 6.0 and 60 ° C for 48 h. After the removal of insoluble materials from the hydrolysate by centrifugation (11,000 rpm, 15 min, Beckman rotor 14), a solution containing 7.2 g of total sugar was obtained. The final concentration of the nutrients added to the solution was 0.2% for peptone, 0.3% for yeast extract, 0.1% for potassium phosphate (monobasic), 0.05% for magnesium sulfate, and 0.2% for calcium carbonate. About 50 mL of the solution supplement with each of the nutrients were placed into five 250 mL shake flasks and sterilized under the above conditions. After cooling, 2 mL of the seed culture of Candida guilliermondii (KCCM 11960) was inoculated into the medium in the flask. Cultivation was carried out at 30 ° C for about 48 h. After cultivation, the yeast cells were removed by centrifugation (11,000 rpm, 15 min, Beckman rotor 14) and the supernatant liquid containing 5.8 g of sugar was obtained. The solution was decolorized with active carbon and was then desalted on columns of cation (Amberlite IR 120 sodium form, Sigma- Aldrich) with anion (Amberlite IRA-400, Sigma-Aldrich) exchange resins. The resulting sugar solution was concentrated to a syrup using a vacuum rotary evaporator. Hot absolute ethanol was added to the syrup to reach a concentration of about 80% ethanol. After the seeding of crystalline MGM and cooling, the MGM was crystallized. The crystals formed were isolated by centrifugal filtration and crystalline MGM was obtained.
Thin layer chromatography was carried out according to the McCleary method (MaCleary, 1982). The sugar sample was dotted on a plate of Merck DC-Alufolien Kiesel gel 60 (Merck, Kenilworth, NJ, USA) and developed using a solvent system of 1-propanol : nitromethane : water (5:2:3, v/v) for about 4 h at room temperature. The sugar on the plate was revealed by heating the plate at 120 ° C for about 10 min after spraying it with 30% H2SO4-ethanol.
Saccharides were hydrogenated into their corresponding sugar alcohols by treating aqueous sugar solutions with sodium borohydride for 2 h at room temperature. The resultant sugar solutions were treated with Amberlite IR-120 sodium form (H + ) to decompose the excess sodium borohydride and remove the base. They were then evaporated with methanol to remove boric acid.
The sugar was methylated using the methods of Ciucanu & Kerek (Ciucanu & Kerek, 1984). The methylated sugar was hydrolzyed in 10% trifluoroacetic acid, hydrogenated with sodium borohydride and acetylated with an equal mixture of pyridine and acetic anhydride. The resultant alditol acetate was analyzed using 3% ECNSS-M column (Shinwa Chemical Industries Ltd., Kyoto, Japan) on a Gas Chrom Q column (Shinwa Chemical Industries Ltd., Kyoto, Japan) and a OV- 210 Spelcoport column (Shinwa Chemical Industries Ltd., Kyoto, Japan) at 190 ° C.
Oligosaccharide was hydrolyzed in 10% trifluoroacetic acid (in an ampoule) by heating at 100 ° C for 2 h. The hydrolysate was evaporated to dryness on a rotary evaporator. The resultant sugars were converted into their alditol acid derivatives and analyzed using gas liquid chromatography paper (Kusakabe et al., 1977) on a 3% ECNSS-M column (Shinwa Chemical Industries Ltd., Kyoto, Japan).
Results and Discussion
Fig. 1 shows the course of cultivation. There was a rapid increase in the production of the enzyme at about 60 h after the beginning of cultivation and the mannanase activity in the culture filtrate reached maximum at the 72 h-cultivation. After the 72 h-cultivation, mycelium was filtered off through a Buchner funnel with Toyo Roshi No. 2 filter paper. The resulting filtrate was then dialyzed against a 4-fold volume of distilled water and the dialyzed solution was used as the mannanase solution for the following experiments.
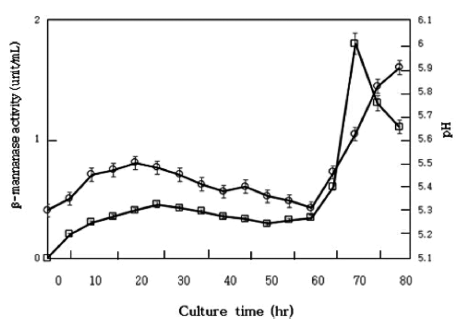
The konjac (2 g), which contained 1.02 g of total sugar (with 0.64 g of mannan), was hydrolyzed with 20 mL of the enzyme solution at pH 6.0 and 60 ° C for 48 h.
Fig. 2 shows TLC of the time-course of hydrolysis of konjac with the enzyme solution. At the reaction time of 48 h, the hydrolysis resulted in monosaccharides and MGM. Next, MGM was prepared from the enzymatic hydrolysate of konjac without using chromatographic separation techniques. I came up with the combination of the hydrolysis of konjac using the crude enzyme and the elimination of monosaccharides from the resultant hydrolysate by selective fermentation with yeast.

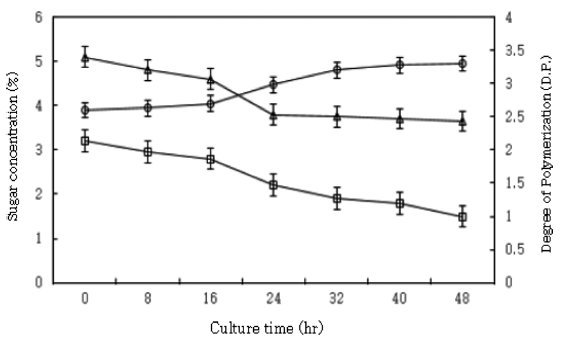
I tried to find a yeast strain capable of metabolizing monosaccharides in enzymatic hydrolysate and of leaving MGM in the hydrolysate; thus, Candida guilliermondii was selected. The time-course of the decrease of sugar in the yeast cultivation was followed by the Somogyi method (Somogyi, 1945). Fig. 3 shows TLC of the time-course of yeast cultures. After 48 h of cultivation, the yeast strain digested monosaccharides but left MGM in the medium. Fig. 4 shows the course of yeast cultivation. As shown in Fig. 4, the sugar content decreased over time until about the 24th h, but no remarkable decrease was observed thereafter. After 48 h of cultivation, the total sugar content fell from 5.2% to 3.7%, and the average degree of polymerization (D.P.) rose from 2.6 to 3.3.

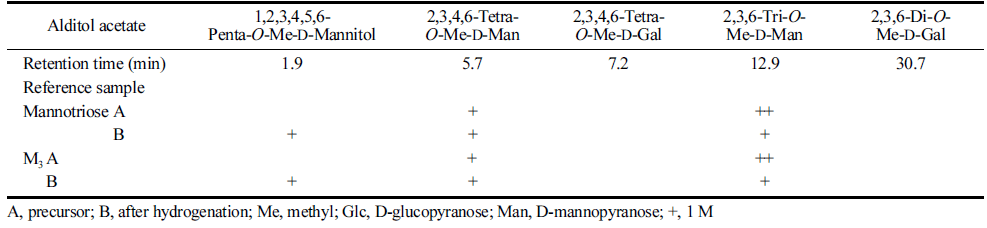
Crude crystals of MGM were recrystallized twice from about 80% aqueous ethanol and 3.8 g of crystalline MGM was obtained. Table 1 shows the results of the methylation analysis of MGM. Evidence supporting this structural interpretation was also obtained by methylation analysis (Table 1). The methylation analysis of MGM revealed 2,3,6-O-Me-Glc (1 M), 2,3,4,6-O-Me-Man (1 M), and 2,3,6-O-Me-Man (1 M). The methylation of the corresponding hydrogenated derivative revealed 2,3,6-O-Me-Glc (1 M), 2,3,4,6-O-Me-Man (1 M), and 1,2,3,5,6-O-Me-Mannitol (1 M). This result indicates that MGM has a 1,4-linkage and the identified O-D-mannopyranosyl- (1→4)-O-D-glucopyranosyl-(1→4)-D-mannopyranose.
|
Conclusion
Previous papers (Park, 2006) reported the specificity of β- mannanase from Trichoderma sp. for Amorphophallus konjac glucomannan. Five types of oligosaccharides were isolated from the hydrolysate of konjac using purified β-mannanase from Trichoderma sp. The enzyme produced mannobiose. It is suggested that β-1,4-linked β-D-mannopyranose residues in the glucomannan were hydrolyzed at the beginning of the reaction and the resultant mannooligosaccharides were further hydrolyzed to produce mannobiose. The enzyme is capable of hydrolyzing both mannotriose and mannotetraose into mannose and mannobiose, but cannot hydrolyze mannobiose further. However, β-mannanase of Xylogone sphaerospora cannot produce mannobiose. Also, the enzyme did not produce glucomanno-oligosaccharides that have a glucose residue at the terminal, but produced glucomanno-oligosaccharides that have a mannose residue at the non-reducing terminal. Moreover, the enzyme did not produce free glucose and cellobiose. These results verify the observation that the enzyme does not have either cellulose or β-glucosidase activity. The strain produced β-mannanase extracellularly and the enzyme system directly attacked the glucomannan in the konjac. Moreover, the optimum pH and temperature for the mannanase activity were pH 6.0 and 60 ° C, respectively. The final products of the digestion of glucomannan by the enzyme system included monosaccharides and MGM, with few other oligomers. I was able to eliminate the monosaccharides by selective fermentation with yeast and to prepare MGM without using any chromatographic techniques. Although the main products were MGM and D.P. (Degree of Polymerization) 4 glucosyl mannooligomer at the 24 h reaction, D.P. (Degree of Polymerization) 4 glucosyl mannooligomer hydrolyzed to mannose and MGM at the 48 h reaction. In addition, it was assumed that the enzyme system may also attack the non-reducing terminal mannose with the lapse of the 48 h reaction. In conclusion, the combination of the hydrolysis of konjac by the enzyme system of Xylogone sphaerospora and the elimination of monosaccharides from the resultant hydrolysate with Candida guilliermondii was suitable for the preparation of MGM from konjac. The oligosaccharide structure was elucidated by the methylation method.







