Introduction
Recent food safety crises by microbial contamination have alarmed consumers who require more ‘natural’ food products with minimal processing and contain fewer additives. These demands have obligated the food industry to adopt new technologies for food preservation (Smelt et al., 2001). Nonthermal methods is one of the minimal process technology, such as irradiation, pulsed electric fields, cold plasma, pulsed light, and high-pressure processing (HPP) (Jayaprakasha & Brueckner, 2000; Yuste et al., 2001). Thermal treatment of foods can have negative effects, not only with respect to the loss of nutrients such as minerals and vitamins, but also the formation of off-flavors and alterations in pigment and texture. Thus, non-thermal processes represent a realistic alternative to traditional thermal processing in the food industry (Douzals et al., 1996; Wennberg & Nyman, 2004).
The HPP system, which comprises a pressure vessel and a pressure-generating device, first requires the product to be loaded into the vessel, which is then sealed. The product is then pressurized, usually using water to generate high hydrostatic pressure. Water is pumped into the vessel from the bottom until the pressure inside the vessel reaches the desired level. At that point the pumping is stopped, the water valves are also closed, and the pressure is maintained, with no more energy being needed (Mertens, 1995; Smelt, 1998; Patterson et al., 2007). HPP is an attractive and relatively gentle method of not only preserving food but also eliminating pathogenic and spoilage microorganisms (Garriga et al., 2004). This technique inactivates the microorganisms by damaging cell membranes, denaturing enzymes, and causing changes in cell morphology (Hoover et al., 1989). The critical factors influencing HPP are the type of food being treated, and the pressure, temperature, and treatment time. HPP involves increasing the pressure and temperature of the food matrix so as to result in microbial destruction. Similar to thermal inactivation methods, pressure does not affect one specific site, but acts on various targets depending on the pressure applied. Since temperature almost always plays a role in pressure treatments, the mode of action of heat is briefly addressed (Cheftel & Culioli, 1997; Rendueles et al., 2011).
As mentioned above, HPP treatment causes morphological changes. For example, the volume of yeast cells gradually decreases when they are subjected to HPP at 250 MPa, and these cells do not completely recover to their original volume when they are returned to normal ambient pressure. A pressure of 200 MPa will damage cell walls and mitochondria (PerrierCornet et al., 1995). Many studies have also found that pressures in the range of 300-600 MPa can inactivate many fungi and vegetative bacteria (Smelt, 1998), while at 400 MPa the shape of Saccharomyces cerevisiae cells is slightly altered (Shimada et al., 1993). Pressures of up to 900 MPa are known to inactivate the vegetative bacteria in food (Smelt et al, 2001). The first commercial, comparatively successful food application of HPP was developed in the 1990s for high-acid jams (Mertens, 1995). The HPP market has now extended to a range of pressure-treated products, including fruit preparations, fruit juices, rice cakes, and raw seafood. The aim of HPP for these products is to reduce the number of vegetative bacteria, molds, and yeasts (Smelt, 1998; Yuste et al., 2001; Patterson et al., 2007).
HPP is an attractive food treatment that renders food safer and extends its shelf life, while retaining many of its original qualities and healthy attributes, and thus has many potential applications. Therefore, the purposes of this study is to investigate the effect of HPP on a sterilization and microbiological shelf-life of commercial beef patties by varying the pressure level (200, 300, 400, and 500 MPa), treatment time (0-10 min), and storage period (0-16 days at 4 ° C), and to assess the potential of HPP treatment for use on a commercial scale.
Materials and Methods
Commercially available seasoned beef patties were purchased from the local market (Lotte Co. Ltd., Seoul, Korea). The meat pieces of approximately 50 g were prepared for HPP treatments and assigned randomly to the various treatments, and then vacuum packed in polyamide polyethylene bags. Samples for microorganism evaluation were stored at 4ºC during storage period (4, 7, 10, 13, and 16 days), and nontreated control samples were immediately prepared for microbial analysis.
HPP treatment of beef patties was performed using a high hydrostatic pressure food processor (Frescal MFP-7000, Mitsubishi Heavy Industries, Tokyo, Japan). Samples were placed in a pressure vessel submerged in a hydrostatic fluid medium and pressurized at 200, 300, 400, and 500 MPa for 2, 4, 6, 8, and 10 min (according to the experimental treatment regime). An initial temperature of hydrostatic fluid was 23 ± 2 ° C. The rate of pressurization was 5-7 MPa/s, and the pressure in the chamber was released within 10 s. Non-treated control samples were maintained under atmospheric pressure at 4 ° C while the other samples were being treated. The temperature in the sample chamber was monitored during processing. Compressive heating during pressurization led to a temperature variation of ± 3 ° C.
The pH values of the samples were measured immediately following HPP treatment, and again after 4, 7, 10, 13, and 16 days of storage. In order to measure the pH, 5 g portions of the meat pieces were assigned to each treatment regime and then blended with 50 mL of distilled water using a stomacher (Model 400, Seward, Thetford, UK). A glass pH probe electrode was placed in the center of the blended sample and the pH was measured using a pH meter (Orion 310, Orion Research Inc., Boston, MA, USA). The average of three measurements was calculated for each sample.
After HPP treatment, the samples were placed into sterile saline solution and pummeled for 120 s at 230 rpm with a stomacher (Model 400, Seward, Thetford, UK). Serial dilutions were prepared with sterile saline. Each of diluted sample (0.1 mL) was spread on plates in duplicate and on 3M Petri film (3M Microbiology, St. Paul, MN, USA) in triplicate. Plate count agar medium (Difco Laboratories, Detroit, MI, USA) was used for aerobic microorganisms. After incubating the plates for 48 h at 37 ° C, those on which 30-300 colonies had formed were counted, with the results quantified as the number of CFU per gram. Reductions of bacteria were calculated as the number of survivors (N) relative to the initial number of microorganisms (N0) after HPP treatment (i.e., log N/N0). Samples for microorganism evaluation were stored at 4ºC during storage period (4, 7, 10, 13, and 16 days), and nontreated control samples were immediately prepared for microbial analysis. All experiments were conducted in triplicate and data are expressed as mean and S.D. values. The inactivation data were analyzed using Microsoft Excel 2007 (Microsoft, Redmond, WA, USA) for checking their significant differences (p<0.05).
Results and Discussion
Fig. 1 shows the inactivation effect of HPP treatment on microorganisms in beef patties as a function of pressure level and treatment time. In HPP treatments at 200 MPa, little inactivation (0.16 log) was observed for a treatment time of 10 min, However, the viability of the microorganisms decreased with increasing treatment time and as the pressure increased from 300 to 500 MPa. HPP treatments during 2 and 10 min at 300 MPa produced 0.61 and 1.16 log reduction, respectively, and HPP at 400 MPa produced 1.11 and 1.69 log reductions, respectively. The treatment for 2 and 10 min at 500 MPa produced 2.73 and 3.86 log reductions, respectively. These results were similar to those obtained from HPP treatment on beef muscle by Cheftel & Culioli (1997) and McArdle et al. (2010). They found no difference in cell counts between HPP treatment at 130 MPa and the non-treated control condition, and a reduction of about 2.5 log at HPP with 520 MPa. Thus, it seems that the application of a higher pressure during HPP will increase the cell reduction, which translates to a huge reduction in bacteria in the meat (Shigehisa et al., 1991).

The mechanism underlying HPP-related inactivation is killing or injuring cells. HPP does not alter covalent bonds, and so the molecular structure remains intact within the range of pressures used in food processing. Interaction between ionic bonds and hydrophobic are unaffected, thus maintaining the secondary and tertiary structures of proteins (Knorr, 1995). In meat science, HPP is mostly used as a method of preservation, using pressures in the range 100-800 MPa; meat products are mainly treated at 300-600 MPa, which is sufficient to inactivate vegetative cells. The pressure causes damage to many aspects of the cell simultaneously, including the cell membrane, cell wall, proteins, enzymes, and genetic mechanisms. Accumulated damage disrupts the cell’s ability to repair itself, leading to death (Hendrickx et al., 1998; Aymerich et al., 2008; Rendueles et al., 2011). Besides the pressure applied, inactivation of microorganisms depends upon the type of microorganism, the treatment time, the food composition, pH, temperature, and water activity (McArdle et al., 2010).
The growth changes of microorganisms in beef patties subjected to the various pressure level (200, 300, 400, and 500 MPa) and treatment time (0-10 min) for HPP were investigated during 0-16 days of storage at 4 ° C (Fig. 2). HPP treatment had a significant effect on the inactivation of microbial growth in beef patties. The initial (non-treated control) bacterial cell count was 7.9×10 4 (CFU/g) and 16 days of storage at 4 ° C following HPP at 200 MPa yielded similar cell numbers (Fig. 2A). Under 300 MPa condition, the number of cells was slightly lower than the control level. After 4-10 min of HPP at 300 MPa, the cell number reached approximately 4.8 log CFU/g after 10 days of storage (Fig. 2B). However, during 16 days of storage, the viable cell numbers in beef patties treated with 400 and 500 MPa were substantially lower than those in the non-treated control (Fig. 2C and 2D). The control count reached 6.6 log CFU/g after 16 days at 4ºC, but after the same storage time, that numbers for HPP-treated samples of 400 and 500 MPa were 4.7 and 3.1 log CFU/g, respectively.
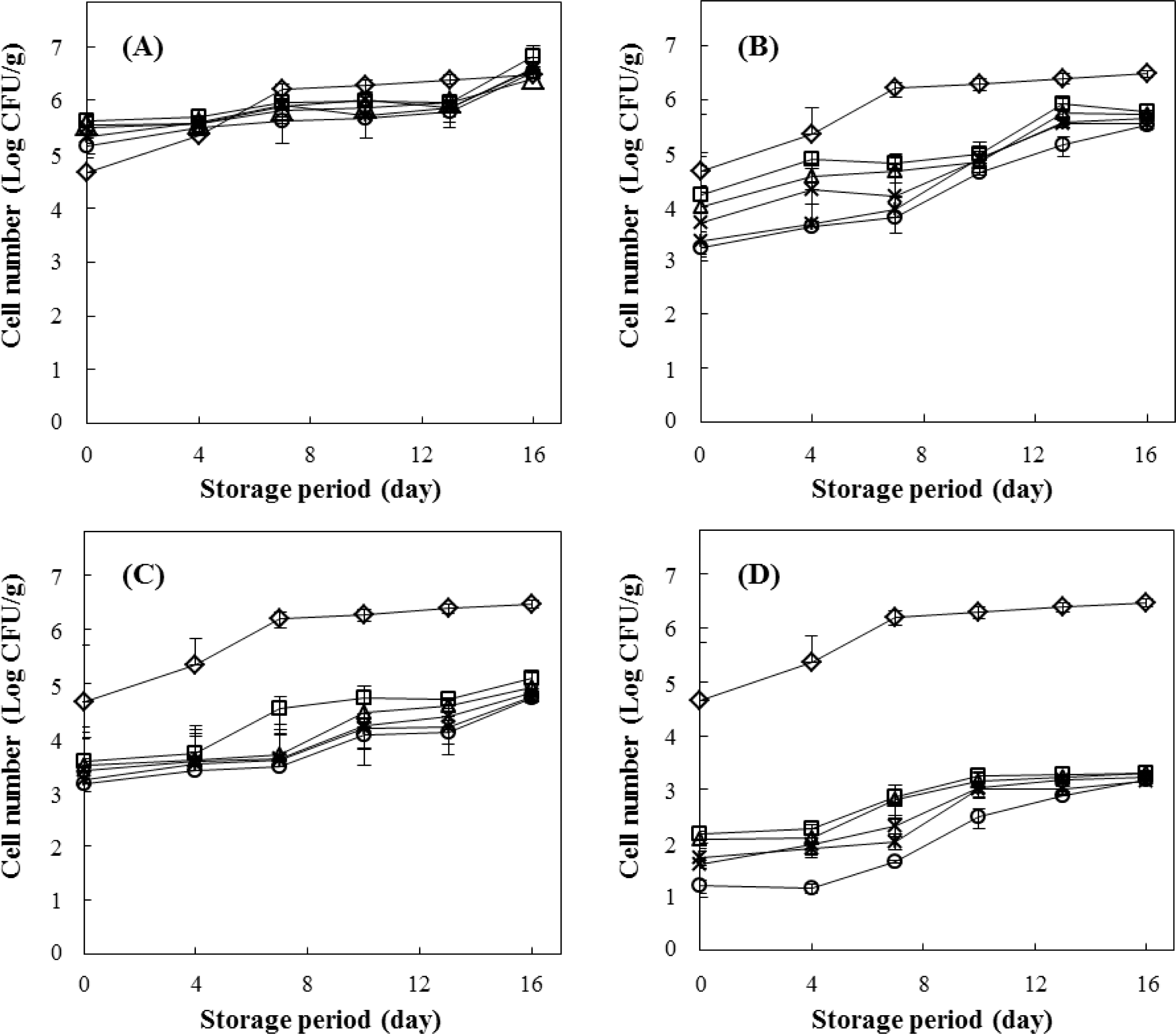
Our results indicate that the HPP treatment of > 400 MPa to beef patties for 2-10 min is significantly effective in reducing the number of viable microorganisms therein and extending the shelf-life. Garriga et al. (2004) reported that 4 log reduction in cell counts was obtained after HPP treatment of marinated beef loin at 600 MPa for 6 min, with no recovery during the storage period at 4 ° C. Montiel et al. (2012) also reported that total counts of viable cells in beef carpaccio after HPP at 450 MPa for 5, 10, and 15 min were at lower than those in the control for 30 days.
The pH changes of beef patties subjected to the various pressure level (200, 300, 400, and 500 MPa) and treatment time (0-10 min) for HPP were investigated during 0-16 days of storage at 4 ° C (Fig. 3). The HPP pressure and storage period significantly affected the pH values. The pH of the non-treated control samples was about 5.71. After HPP treatment, the slight increase in the pH values was observed and the pH values were approximately 5.83-5.86, 5.92-6.02, 6.04-6.12 and 6.17-6.28 in HPP-treated samples of 200, 300, 400, and 500 MPa, respectively (Fig. 3A-D). During the 16 days of storage, the pH in the non-treated control and the samples of 200 MPa increased to about 6.04 with increasing storage period (Fig. 3A). However, the pH values of the samples treated with 400-500 MPa were maintained at initial levels throughout the storage period, regardless of treatment time for HPP (Fig. 3C-D).
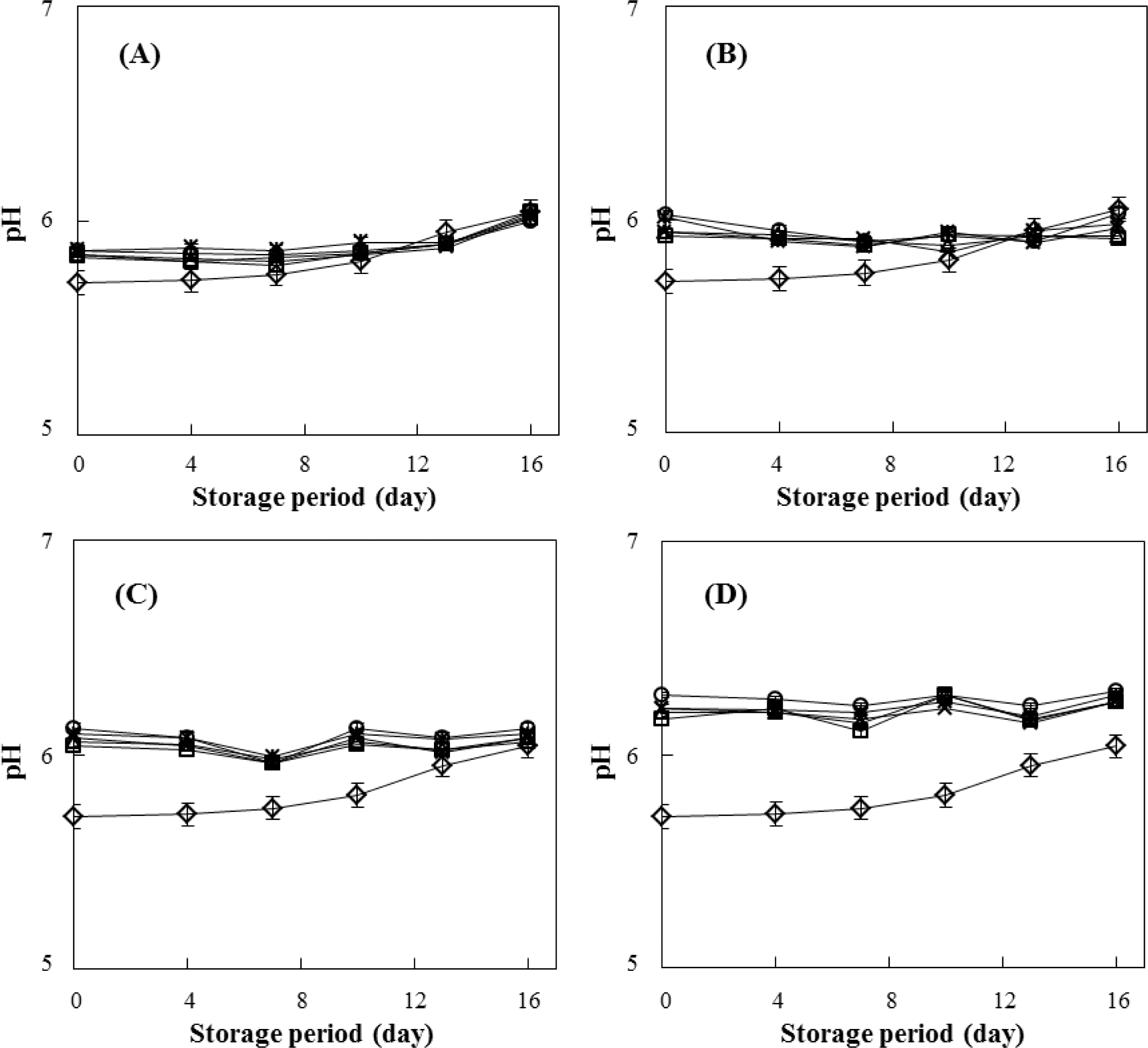
Similar results were reported in minced pork (Cheah & Ledward, 1996) and Indian white prawn (Bindu et al. 2013) treated with HPP. Cheftel & Culioli (1997) suggested that increase of pH in HPP-treated samples is the result of a reduction in the available acidic groups in meat due to a change in protein conformation (i.e., denaturation). In addition, during storage period, an increasing trend of the pH values in the non-treated control and 200 MPa may due to the production of volatile base compounds by microbial growth (Bindu et al., 2013). As shown in Fig. 2, the HPP treatment of > 400 MPa to beef patties was effective in reducing the number of viable microorganisms and extending the shelf-life.
Summary
HPP is an attractive method that supplies safe food and extends its shelf-life by eliminating pathogenic and spoilage microorganisms, while retaining many of its original qualities and healthy attributes. The results of the study have confirmed efficacy of a sterilization and microbiological shelf-life extension by HPP treatment as preservation method on commercial beef patties. Therefore, this method could be developed to enhance meat hygiene and extend the shelf-life of commercial meat product such as beef patties. However, further studies are needed in order to better understand the physiochemical and morphological changes occurring in pressurized meat, and effect of these changes on sensory characteristics of beef patties.







