Introduction
The growth in convenience food industry has been attributed to various reasons, including increasing number of working women and single household, variable meal time, shortage of time, and lack of cooking skills (Kanzler et al., 2015). Furthermore, single servings and products with small pack size are more in demand, which has boosted the growth of convenience food industry. In this context, home meal replacement (HMR) has emerged as a great solution.
To manufacture processed meat products, the meat processing industry has frozen and thawed only raw meat untill date. The meat products processed from raw meat require a long time for thawing, so a considerable amount of time is lapsed before they can be served during meals. Apart from this, there may be problems associated with the cooking aspect of these products, such as irregular heating, inadequate thawing, and microbial contamination. Frozen convenience food requires minimum heat treatment before they could be served in meals. In summary, pre-thermal treatment must be used in meat processing industry for cooking frozen meat products either partially or completely; these are convenience foods that have undergone extensive processing.
Hot water blanching has been used as a sub-cooking method in this research study. It was expected that blanching would inactivate enzymes and decrease microbial activity (Cano, 1996). In addition, blanching treatment prevents the development of unpleasant odors in meat (Gonçalves et al., 2009). Furthermore, Individual Quick Freezing (IQF) technology is used to minimize tissue deterioration of blanched beef, a process that is caused by ice crystal formation in beef that is frozen and stored. With the help of IQF technology, food samples are frozen through ultra-fast freezing in an air blast freezer at an extremely low temperature range of -30 to -40 °C; this process leads to the formation of fine intracellular ice crystals and diminishes thawing loss (Zhou et al., 2010; Jo et al., 2014). Frozen storage not only helps in prolonging the shelf life of meat but also helps in preventing microbial and chemical changes in the meat (Lawrie & Ledward, 2006).
In this research, beef was chosen as an ingredient as it is widely used in Korean cuisine and generates tremendous public interest (Lee et al., 1990; Yoon & Woo, 1999). The aim of this research is to devise an optimum storing condition for frozen beefs that could be used in preparing HMR products.
Materials and Methods
The fresh beefs (round of beef) were purchased from a wholesale market in Suwon, Korea. Plate count agar (PCA) was purchased by Difco Laboratories (Detroit, MI, USA).
Before treatment, the fatty portions of raw beefs were removed. Then, the remaining lean meat of raw beefs was washed and cleaned with distilled water. Parallel to the fiber direction, the beefs were sliced, each slice having a dimension of 5.0×0.5×0.5 cm 2 . The beef slices were subjected to hot water boiling blanching for two minutes. The ratio of beef slices to hot water was about 1:4. After blanching, these thermally treated beefs were immediately soaked in cold water to reduce the heat of beefs. The beefs were cooled and then spin-dried for 3 minutes in a spin-dryer. This process helped in removing water from the exterior of beefs. Thermally treated beefs were frozen individually through individual quick freezing (IQF) technology at -40 °C temperature. For this purpose, the IQF freezer (Chunil food Ltd., Seoul, Korea) was used. Then, beefs were packed using two packing methods: vacuum packaging and air-containing packaging. Vacuumpacked beefs were packed using 200 g of frozen beefs in every package; the vacuum package machine was operated for packaging these beef items. On the other hand, air-containing packed beefs were packed using 200 g of same frozen beefs in a package: air-containing package machine was operated for packaging these beef items. After packaging, all the packed samples were stored for 9 mon in a freezer at the following temperatures: -12 °C, -18 °C, and -24 °C respectively, which means general temperature of freezing storage for consumer (-18 °C) and its above and below. Once in every three months, the samples were thawed by heating in a microwave (400 W, 5 min). Then, physico- chemical properties of these samples were evaluated.
The thawing loss of thawed beefs was calculated by taking into Consideration the known weights of beefs before and after thawing. Thawing loss was defined as per the following formula:
The color changes of samples were measured using a colorimeter (CR-300, Konica Minolta Sensing Inc., Kyoto, Japan) and calibrated with the white standard plate (L*=77.1, a*=2.1, b*= 2.2). The CIE (International Commission on Illumination; abbreviated CIE for its French name) L*, a*, and b* values were measured as indicators of L* (brightness), a* (redness), and b* (yellowness), respectively. For the measurement of color, three pieces of samples were attached to the long direction. Total color differences (ΔE) were calculated as the color difference between the raw beef and treated samples using the following equation:
Two grams of a sample was added to 18 mL of distilled water and homogenized at 12,000 rpm for 1 min using a homogenizer (HP-91, SMT Co. Ltd., Tokyo, Japan). The pH of homogenized samples was measured using a pH meter (Orion 3 star, Thermo scientific, Waltham, MA, USA).
All the cuboids (5.0×0.5×0.5 cm 2 ) formed samples were placed individually on a flat board in the vertical direction against the muscle fiber. The hardness of samples was measured using a texture analyzer (CT3-1000, Brookfield Co. Ltd., Middleboro, MA, USA) having a TA3 probe made from stainless steel; this probe was used for cutting. The samples were measured on the basis of the following parameters: compression type, 10 kg force load cell, 300 g trigger load, 2.5 mm/s test speed, and a target distance 5 mm. The hardness (Maximum peak force, g) was a texture parameter of samples. All these treatments were replicated five times.
Results and Discussion
Thermal treatment is a necessary step to Cooking for meat and makes it digestible (Shabbir et al., 2015). Blanching treatment of beef changed its properties such as hardness, pH and color (Ma & Ledward, 2004). In this research, the hardness of beef increased from 2.7 kg to 4.1 kg with thermal treatment (Table 1). The L * value had an increase, whereas the a * value showed a decline. The b * value didn’t showed any large change, reducing from 11.94 to 11.35. As expectedly, an increase of pH and a decrease of total aerobic bacteria level were observed. These changes were attributed to the unfolding and denaturation of protein through thermal treatment (Shabbir et al., 2015).
During the freezing process, intracellular juice of beefs is expelled by osmosis into the extracellular region. This facilitates the formation of ice crystals, causing juice loss from beefs during thawing. These effects can influence the quality of beefs (Pietrasik & Janz, 2009).
In this research study, all the frozen samples were thawed in amicro wave before evaluating physicochemical properties. Moreover, the extracellular juice generated during thawing was removed. In all the samples, thawing loss increased with increase in temperature of freezing (Fig. 1). It was observed that the tendency that increased thawing storage loss intensified at higher storing temperature. The beefs stored at -12 °C had the highest thawing loss when the samples were packaged by the two packaging methods: vacuum packaging and aircontaining packaging. In vacuum-packed beefs, the samples stored at -12 °C had the highest thawing loss, whereas the thawing loss of samples stored at -18 and -24 °C were less than 1.3%. At the same storage temperatures, atmosphere-packed beefs underwent higher thawing loss than vacuum-packed ones. The samples stored at -12 and -18 °C had higher thawing loss, which ultimately exceeded 10% in the 9-mon duration of freezing. The atmosphere-packed beef samples stored at -24 °C also had around 4% loss in the thawing process, which was comparatively higher than that undergone by vacuum-packed ones.
As only drip loss of thawing process affects the properties of beef, it is possible to enhance the entire quality of meat (Tsukamasa et al., 1992; Yu et al., 2010). Kim et al. (2013) have reported that microwave is the best thawing method for frozen beefs. The thawing losses of frozen beef, which is obtained from the round, were in the range of 1.3-2.5%. Considering drip loss after thawing treatment, vacuum packaging was more effective for freeze storing of beef than air-containing packaging.

It has been reported that 74% of consumers consider the color of a food product as an important property that influences their purchase decisions (Lynch et al., 1986). In this study, Tables 1 and 2 display the instrumental color parameters (L*, a* and b*) for round of raw beef samples and treated samples, respectively. Among all the samples, raw beefs had the lowest L* value and highest a* values. The L* value of beefs increased from 37 to 50, while a* value decreased from 22 to 6 when the samples were subjected to thermal treatment. In the 0 mon, beefs were measured the CIE color values immediately after freezing, packing, and thawing. Similar color values of 0 mon samples were obtained at lower storage temperature, regardless of the type of package. Among vacuum-packed samples, the samples stored at -18 and -24 °C showed lower change in L* value compared to the samples stored at -12 °C samples. In samples backed by both the methods, the ones stored at -12 °C stored samples obtained the highest b* values (p<0.05). After freezing for 9 mon, the b* value of vacuumpacked and air containing-packed beef samples stored at -12 °C was higher than 16, while as the b* value of samples stored at other temperatures was significantly lower than 12 (p<0.05). There was a consistency in a* values of all the samples. As shown in Tables 1 and 2, hot water blanching is the most effective treatment for dealing with color change of beefs. When the beefs were stored at lower temperatures after freezing, there were smaller differences in the color of 0-mon samples than the ones stored at -12 °C (p<0.05). The color of frozen meat changes when it is stored for a consideration period of time. During this period, it is subjected to freezethaw cycles that cause temperature and color changes due to the increasing activity of α-glucosidase and β-N-acetylglucosaminidase in catfish (Benjakul & Bauer, 2001). The frozen meat changes color owing t°Changes in the state of myoglobin: the pigment exists in this muscle tissue of meat products. In the post-mortem stage, the myoglobin of meat exists in the form of oxymyoglobin, a muscle tissue that has red color. When this meat is subjected to thermal treatment, oxymyoglobin is oxidized to metmyoglobin, which has brown color (Warren et al., 1996). As a result, after subjecting the beefs to hot water blanching, the L* values of beefs increased, while a* values decreased. During the storage period, the color changes in the meat were less drastic than the changes witnessed during thermal treatment the drastic changes in color occur as enzymes in the beef get inactivated when subjected to thermal treatment.
1) Control: blanched beefs without freezing V-12: frozen beefs with vacuum packaging stored at -12°C V-18: frozen beefs with vacuum packaging stored at -18°C V-24: frozen beefs with vacuum packaging stored at -24°C A-12: frozen beefs with air containing packaging stored at -12°C A-18: frozen beefs with air containing packaging stored at -18°C A-24: frozen beefs with air containing packaging stored at -24°C.
In this research study, pH increased from 5.67 to 5.78 when the beefs were subjected to thermal treatment (Fig. 2). After blanching the beefs, its pH increased to 5.78. Thereafter, when the beefs were frozen and thawed, the pH increased to a value greater than 5.8. When the beefs were stored under frozen conditions for a considerable period of time, the pH of all the samples showed a tendency to decrease the decreases in the pH of all the samples were similar in the first three months of storage. Interestingly, the pH of frozen beefs started increasing again from the sixth month. In case of air-containing samples, the pH values of samples after 6 mon of storage were similar with the pH values of samples in the 0-mon. It has been reported that the pH of thermally treated meat is higher than that of raw meat, which is in turn attributed to the concealing acid groups of unfolding proteins (Ma & Ledward, 2004). Yang et al. (1989) had reported that the pH of frozen pork and beef declined initially when they were subjecting them to freezing storage however, in the later months of storage period, the pH of frozen pork and beef increased again due to denaturation of proteins and lipids. This finding was in good agreement with the results of our study. In addition, Boles & Swan (2002) had reported increase after decline of pH with freezing storage, assuming the relevance with purge and pH of beef. The pH of beef had a decline with an increase of purge. The variation of purge in frozen beef during freezing storage may affected to pH.
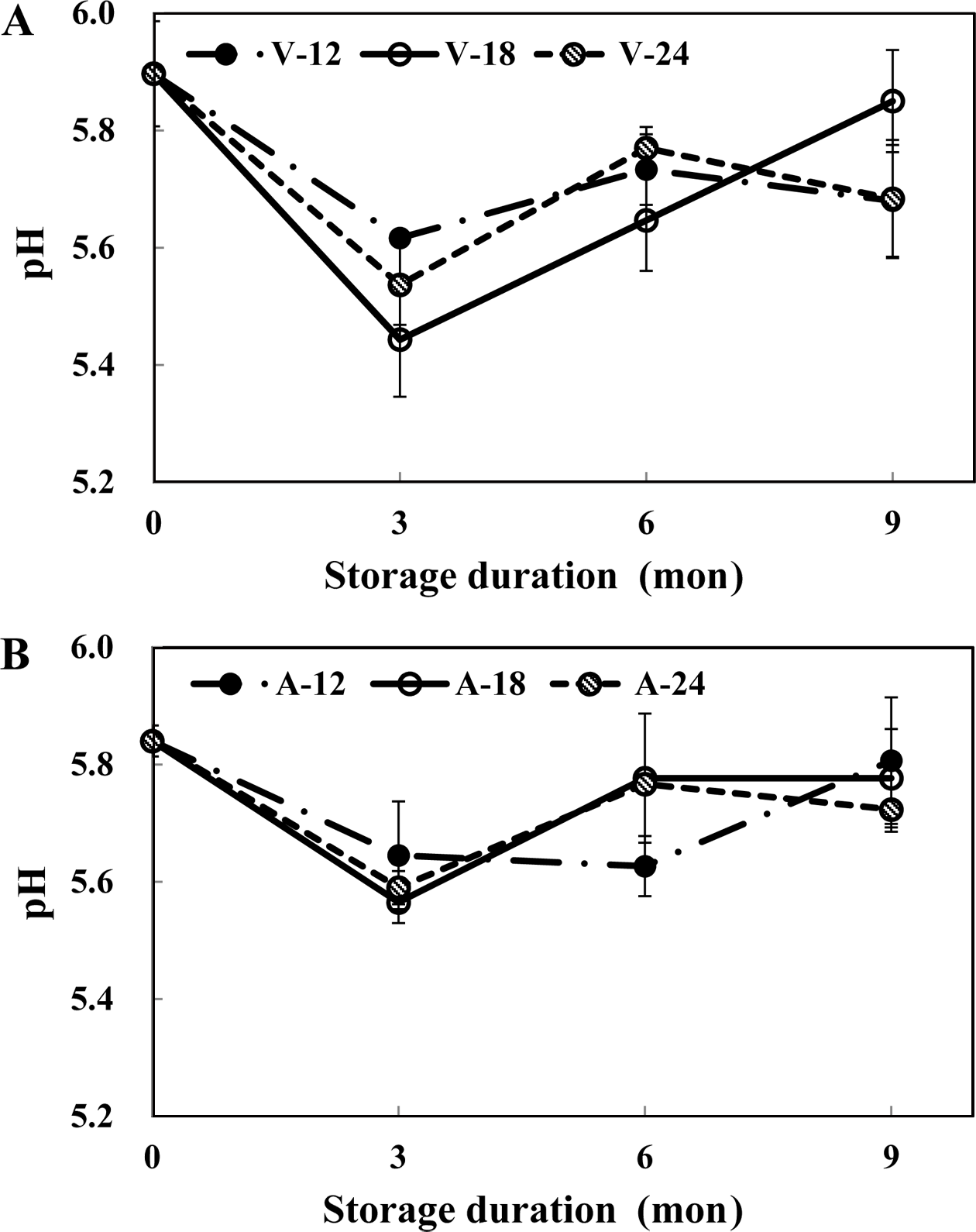
Fig. 3 depicts the shear force of thawed beefs that were subjected to freezing storage for a specific duration of time. Raw beef weighing 2.7 kg was subjected to blanching treatment changed shear force of beefs weighing 4.1 kg. After freezing and thawing, shear force of beefs was increased again to 5.0 kg. During freezing storage, shear force was steadily increased. Shear forces of beefs stored at -18 °C had the lowest values; the values of shear forces of beefs were similar to those of samples stored in the 0-mon. These samples were first packaged using any of the two different packaging methods used in this study. Vacuum-packed samples that were stored at -18 °C for 9-mon exerted about 5.1 kg for shear force, while the other samples exerted shear forces that were greater than 6.0 kg. But, the vacuum-packed sample stored at -24 °C was an exception in this case; it produced shear force of about 6.5 kg. After storing the samples for six month, it was found that there was little difference between the shear force values of samples stored at -12 °C and -24 °C.
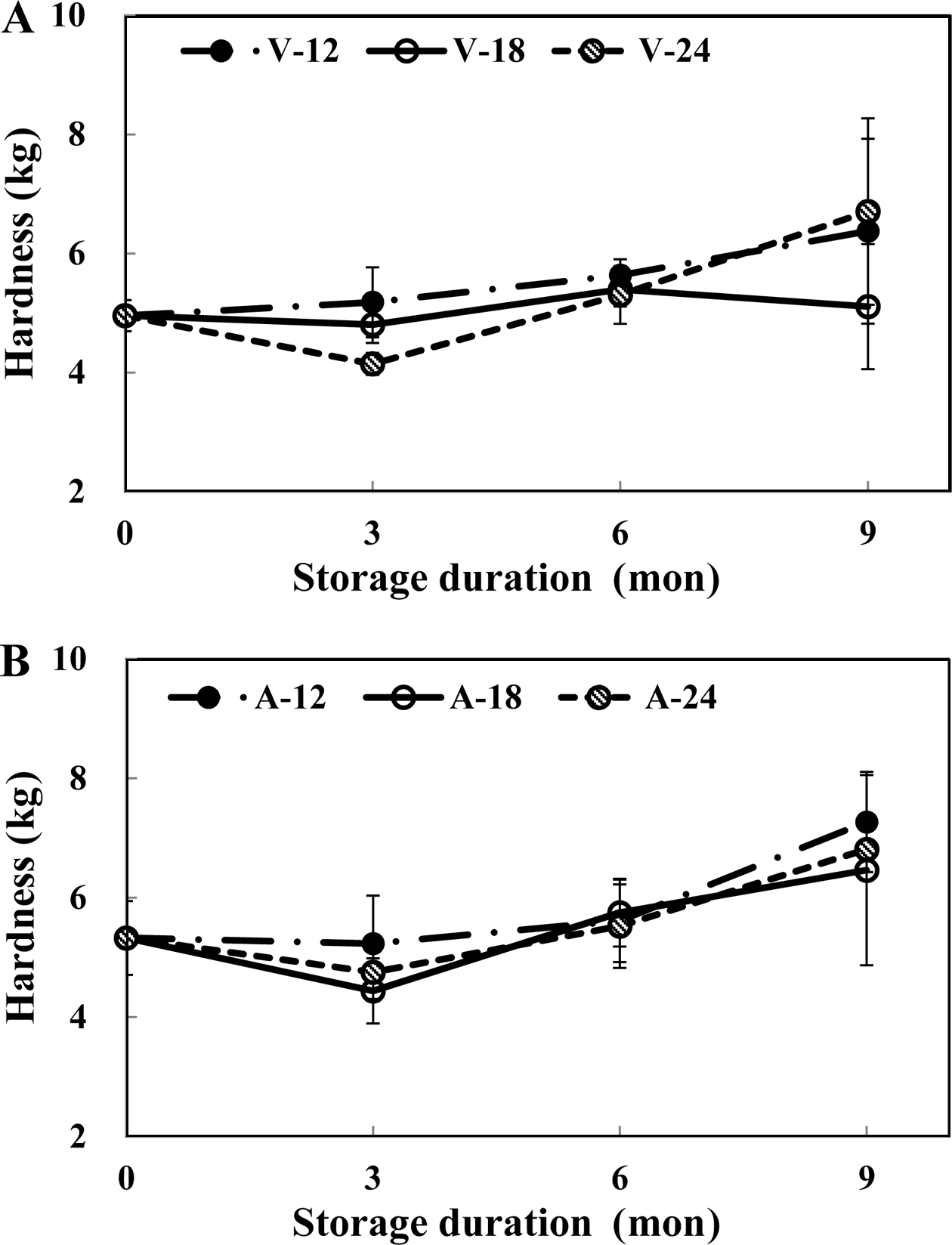
It have been observed the shear force of meat increased with increasing temperature, which has been attributed to the structural changes of myofibrillar components and shrinkage of connective tissues (Ma & Ledward, 2004). Furthermore, Bhattacharya et al. (1988) have reported that an increase in the shear force of beef occurs with the passage of time provided the beefs are subjected to freezing. Moreover, they also found that the value of shear force exerted by the vacuum packed samples was lower than that exerted by air-containing packed samples. These results were similar to the findings of this research study.
In the context of HMR, it is necessary to ensure that pretreated beefs maintain their shear force during freezing storage without getting hardened. In case of HMR products, vacuumpackage and storage at -18 °C is the proper storage condition. This would maintain the shear force of pre-heated and frozen beefs.
Conclusions
In this study, the IQF method was applied on beef and determined whether frozen HMR products could be prepared from beefs. In the 9-mon period of freezing storage, it was found that vacuum-packaging and -18 °C were the ideal conditions for storing beefs as it minimized the changes of physicochemical properties. Furthermore, it was convenient to store beefs at -18 °C as it is already adopted as the general temperature for storing frozen foods.







