Introduction
Oxidative stress results from an excess of reactive oxygen species (ROS) generated due to an imbalance between ROS levels and the antioxidant defense system (Lushchak, 2014). Excessive oxidative stress is associated with the development of various chronic inflammatory diseases, including prostate cancer, atherosclerosis, and vascular disease (Sies et al., 2017). Furthermore, chronic inflammation stimulates the secretion of inflammatory cytokines such as interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α) as well as the production of ROS, thereby triggering oxidative stress (Feghali & Wright, 1997). To effectively treat chronic inflammatory diseases, it is important to reduce the expression of free radical by enhancing the activity of antioxidant enzymes and to suppress inflammation by reducing pro-inflammatory cytokines (Roy et al., 2011;Hwang et al., 2019). Therefore, intervening with antioxidants to reduce free radicals and alleviate oxidative stress is beneficial for the prevention and treatment chronic inflammation (Biswas, 2016).
Natural antioxidants present in medicinal plants are responsible for their powerful redox ability to scavenge free radicals (Ramawat et al., 2009). Due to the potential of various natural resource for health benefits including antioxidant capacities, many countries competed and invested to conserve and secure their domestic biological resources. Especially, the Nagoya Protocol on access to genetic resources and benefit sharing (ABS) in 2014 overheated the international competitions to secure bioresources. The Korean government has also heavily invested in biobanking activity (Lee et al., 2016). The Korea Research Institute of Bioscience and Biotechnology (KRIBB) is Korea’s national biobanking center that secures various domestic research resources, including biological and non-biological resources. Not only aggregating biological resources, KRIBB has set up International Biological Material Research Center (IBMRC) to establish network with global biobanks for international research collaboration and collection of novel specimens. Among them, the Ethnobotanical Database of Bangladesh that is one of the Bangladesh Biobanks collaborates with IBMRC (Lee et al., 2016). Due to the collaboration, diverse Bangladesh biological samples including diverse herbal plants are usable for research purpose in Korea. Bangladesh is renowned among South Asian countries for its rich array of medicinal plants and herbal medicines. It is estimated that more than 500 species of medicinal plants flourish in Bangladesh, with approximately 250 of them finding use in traditional medicine preparations (Uddin et al., 2011). These traditional remedies have historically addressed conditions such as diarrhea, stomachaches, dysentery, wounds, and diabetes. In particular, Bangladeshi medicinal plants from tropical and subtropical regions are recognized for their high-quality and quantity of antioxidants, owing to the intense sunlight exposure (Hanamura et al., 2005).
Many studies have focused on antioxidant and anti-inflammatory effects within the leaves or the entire plant structure, while other components such as bark or stems may also contain bioactive substances (Mahabub-Uz-Zaman et al., 2009;Hossain, 2012;Hasan et al., 2014;Bakhtiar et al., 2015;Pal & Sil, 2023). However, no studies have been conducted to compare the overall antioxidant and anti-inflammatory effects of bark parts of medicinal plants in Bangladesh. In particular, antioxidant compounds contained in the bark of medicinal plants play a role in protecting against the production of free radicals and help alleviate oxidative stress-related diseases (Abeyrathne et al., 2022). Furthermore, it is well known that the bark of medicinal plants is rich in phenolic polymers such as phenolic acid, lignin, stilbene, and tannin, which provide powerful antioxidant properties (Feng et al., 2013;Bouras et al., 2015;Reinisalo et al., 2015;Tanase et al., 2019). Recent studies have highlighted that phenolic compounds derived from woody vascular plants, especially bark, are attractive biological sources to inhibit oxidative stress (Tanase et al., 2019;Venkatesan et al., 2019). These phenolic compounds found in medicinal plant bark are recognized for their antioxidant, anti-inflammatory, immune system-boosting, and anti-aging properties (Ojha et al., 2019). Therefore, this study aims to demonstrate antioxidant and anti-inflammatory effects using methanolic bark extracts of medicinal plants used in Bangladesh, thereby providing broad applicability for the new source.
Materials and Methods
Sodium carbonate (Na2CO3) was purchased from Tokyo Chemical Industry (Tokyo, Japan). Folin-Ciocalteu’s reagent, gallic acid, ascorbic acid (vitamin C), 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), 2,4,6-tri[2-pyridyl]-s-triazine (TPTZ) phosphate-buffered solution (PBS), citric acid, sodium acetate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), FeSO4·7H2O, and FeCl2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2,2'-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) was purchased from Roche (Basel, Switzerland). For cell experiments, Dulbecco’s Modified Eagle’s Medium-high glucose (DMEM), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT), and lipopolysaccharide (LPS) from Escherichia coli O26:B6 were purchased from Sigma-Aldrich. Dulbecco’s phosphate-buffered saline (DPBS) was purchased from WelGENE (Daegu, South Korea). Penicillin-streptomycin solution (P/S) was purchased from Cytiva-Hyclone Laboratories, Inc. (Logan, UT, USA). Fetal bovine serum (FBS) was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Powdered bark extracts from fifteen medicinal plants native to Bangladesh were obtained from the IBMRC at the KRIBB. These medicinal plants were collected from various regions of Bangladesh between March and October 2015 and were taxonomically identified by Md. Salah Uddin, a taxonomist at the Ethnobotanical Database of Bangladesh (Table 1). The collected bark samples were dried in the shade and ground by grinder. Subsequently, 100 g of powdered bark samples was mixed with 1 L of 99.9% methyl alcohol (HPLC grade). The extraction process was carried out using an ultrasonic extractor (SDN-900H, SD-Ultrasonic Co., Ltd., Seoul, Korea) over 30 cycles, involving ultrasonication at 40 KHz and 1500 W for 15 min, followed by a 120 min resting period for each cycle, all performed at room temperature. Following extraction, the filtrates were passed through a Qualitative Filter No. 100 (Hyundai Micro Co., Ltd., Seoul, Korea) and subsequently dried under reduced pressure. The powdered extracts were then obtained by IBMRC at KRIBB, documented in a library, and deposited in the herbarium for future distribution to Korean researchers. After obtaining the samples from IBMRC, the bark extract powders were dissolved in methanol at a concentration of 1000 mg/mL to create stock solutions for the measurements of total phenolics and total antioxidant activities. Subsequently, working solutions were prepared by diluting the stock solutions to the appropriate concentrations using methanol.
The total phenolic content (TPC) of methanol extracts from the bark of 15 medicinal plants of Bangladesh was measured using the Folin-Ciocalteu method (Baek et al., 2021). Briefly, 10 μL of bark extract were mixed with 10 μL of Folin & Ciocalteu’s reagent and 130 μL of distilled water in a 96-well plate. After incubating for 6 min at room temperature, 100 μL of 7% Na2CO3 solution was added into mixture and incubated for 90 min at room temperature. The absorbance was measured at 750 nm using a Multiskan SkyHigh microplate spec-trophotometer (Thermo Fisher Scientific, MA, USA). TPC of each sample was expressed as mg gallic acid equivalents (GAE)/g dry weight.
ABTS radical scavenging assay was investigated using a little modified method (Baek et al., 2021). To make ABTS radical reagent, 1.0 mM AAPH and 2.5 mM ABTS were dissolved in PBS and reacted at 80°C water bath for 40 min in the dark. The ABTS radical solution was filtered using a 0.45 μM PVDF syringe filter and diluted with PBS to adjust the absorbance of 0.700 ± 0.020 at 734 nm. A total 10 μL of bark extract was mixed with 240 μL of ABTS radical solution in a 96-well plate, followed by incubation at 37°C for 10 min. The absorbance was measured at 734 nm by a Multiskan SkyHigh microplate spectrophotometer (Thermo Fisher Scientific). The inhibition percent of ABTS+ was calculated according to the following equation: scavenging effect (%) = (1 - (Asample/Acontrol) × 100%. Where Acontrol is the absorbance of control reaction and Asample is the absorbance of the sample with ABTS+. The antioxidant capacity in samples was expressed as half-maximal inhibitory concentration (IC50) that indicates the concentration of the sample required to scavenge 50% of radicals.
DPPH radical scavenging activity of bark extracts was measured according to the slightly modifi ed method (Baek et al., 2021). DPPH radical solution (0.1 mM) was prepared by mixing DPPH and 80% methanol. The solution was diluted with 80% methanol to adjust the absorbance of 0.700 ± 0.020 at 517 nm using a Multiskan SkyHigh microplate spectrophotometer (Thermo Fisher Scientific). A total of 5 μL of bark extract were mixed with 245 μL of DPPH solution and incubated for 30 min at room temperature. The absorbance was measured at 517 nm using a Multiskan SkyHigh microplate spectrophotometer (Thermo Fisher Scientific). The antioxidant capacity is expressed as percentage inhibition, calculated using the following formula: scavenging effect (%) = (1 - (Asmaple/Acontrol) × 100%. Where, Acontrol is the absorbance of the control and Asample is the absorbance of the sample at 517 nm. The DPPH radical scavenging capacity of bark extracts was calculated as IC50.
Ferric reducing antioxidant power (FRAP) of bark extracts was measured according to a slightly modified method (Benzie & Strain, 1996). FRAP reagent was prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ, and 20 mM ferric chloride hexahydrate (FeCl3·6H2O) solution at ratio of 10:1:1 (v/v), respectively. After mixing FRAP solution, it was kept at 37°C until use. Six μL of each bark extract was mixed with 200 μL of FRAP solution and incubated at 37°C for 4 min in a 96-well microplate. The absorbance was measured at 593 nm using a Multiskan SkyHigh microplate spectrophotometer (Thermo Fisher Scientific). The standard curve linear range was between 0 and 1.0 mM FeSO4. FRAP of bark extract was expressed as mM FeSO4 equivalent (FSE)/g dry weight.
Murine RAW 264.7 macrophages (Korean Cell Line Bank Seoul, South Korea) were cultured in DMEM supplemented with 10% FBS, 1% P/S in a humidifi ed incubator at 37°C with 5% CO2 atmosphere.
Cell viability was measured using MTT assay. RAW 264.7 macrophages were treated with various concentration (0-100 μg/mL) of bark extracts for 24 h. MTT solution (500 μg/mL) was added to the cells and incubated at 37°C for 1 h. After discarding culture media, the formazan crystals were dissolved by dimethyl sulfoxide. Absorbance at 570 nm was measured using BioTek Cytation 5 Image reader (Winooski, VT, USA). Cell viability revealed no significant toxicity at various concentration (0-5 μg/mL) of bark extracts (data not shown). Further experiments were conducted at bark extract concentration under at μg/mL.
The mRNA expression levels of specific genes were evaluated by qRT-PCR. RAW 264.7 macrophages were pre-treated with 1.25, 2.5, and 5 μg/mL of bark extract or vehicle for 6 h, and then stimulated with or without 100 ng/mL of LPS for 3 h. Total RNA extract from macrophages, synthesis of cDNA, and qRT-PCR were performed as previously described (Cao et al., 2023). The primers for RT-PCR were manufactured by Macrogen Co., (Seoul, Korea), and the sequences are listed in Table 2.
Results
Phenolic compounds from medicinal plants are characterized by a benzene ring substituted with hydroxyl groups and exhibit a variety of biological properties, including antioxidant, anti-inflammatory, antiproliferative and antibacterial activities (Albuquerque et al., 2021;Baek et al., 2021). TPC was first performed to investigate which bark extracts of 15 Bangladeshi medicinal plants had the highest phenolic content (Table 3). TPC result for 15 bark extracts ranged from 17.05 to 369.18 mg GAE/g dry weight. Among 15 types of bark extracts from Bangladesh medicinal plants, Albizia odoratissima (369.18±26.47), Engelhardia spicata (309.50±70.70), Xylocarpus moluccensis (357.78±41.41), and Shorea robusta (243.08±93.64) showed the highest TPC. On the other hand, Wrightia arborea (17.05±2.44) and Vitex peduncularis (22.20± 0.37) exhibited the lowest TPC.
The total antioxidant capacity of the 15 bark extracts was assessed using ABTS, DPPH, and FRAP analysis. The antioxidant activities of ABTS and DPPH are expressed as IC50 values (Table 3). Among 15 bark extracts, the highest IC50 values for ABTS radical scavenging activity were observed from E. spicata (60.80±4.31 mg/mL) followed by D. turbinatus (63.42±1.59), S. robusta (63.78± 1.49), and A. odoratissima (68.84±4.79). For DPPH radical scavenging activity, A. odoratissima showed the lowest IC50 values among 15 bark extracts as 181.39±1.91 μg/mL, followed by E. spicata (192.59±2.42), S. robusta (211.87±1.59), B. sexangula (231.28±5.89), and S. declinate (240.12±1.52). In the case of FRAP assay, A. odoratissima and E. spicata also showed the highest reducing power as 5.62±1.03 and 5.50± 0.73 mM FSE/g dry weight, respectively. Therefore, among 15 bark extracts, three bark extracts from A. odoratissima, S. robusta, and E. spicata exhibited the highest total antioxidant activity in all three assays such as ABTS, DPPH, and FRAP.
Pearson correlation analysis was conducted to examine the relationship between TPC and three antioxidant assays such as ABTS, DPPH, and FRAP assay (Table 4). TPC assay had a significantly positive correlation with FRAP assay (r = 0.872, p<0.01). Also, ABTS assay showed the highest significant correlation with DPPH assay (r = 0.972, p<0.01) compared to other assays correlation. However, ABTS and DPPH assays had a negative correlation with TPC and FRAP assays (ABTS&TPC; r = -0.664, p<0.05, ABTS&FRAP; r = -0.599, p<0.050, DPPH&TPC; r = -0.604, p<0.05, DPPH &FRAP; r = -0.549, p<0.05). Since lower the IC50 value indicates stronger antioxidant activity, negative correlations suggest that higher TPC values result in lower IC50 values of DPPH or ABTS scavenging activity. Thus, this result demonstrates a significant negative correlation between TPC and IC50 values of DPPH or ABTS scavenging activities.
| Antioxidant activity | TPC | ABTS | DPPH | FRAP |
|---|---|---|---|---|
|
|
||||
| TPC | 1 | -0.664 (p<0.01) | -0.604 (p<0.05) | 0.872 (p<0.01) |
| ABTS | 1 | 0.972 (p<0.01) | -0.599 (p<0.05) | |
| DPPH | 1 | -0.549 (p<0.05) | ||
| FRAP | 1 | |||
To compare TPC and total antioxidant capacity of bark extracts of 15 medicinal plants from Bangladesh, a heat map analysis was performed (Table 5). The heat map used red to represent the highest levels of TPC and antioxidant capacities, transitioning to light red for lower levels, and white for average levels. Among 15 bark extracts, A. odoratissima, E. spicata, and S. robusta showed the highest of TPC and antioxidant capacities. Among the rest, B. sexangular, D. turbinatus, S. apetala, and X. moluccensis exhibited significantly outstanding TPC and antioxidant capacities. On the other hand, V. peduncularis and W. arborea revealed the lowest levels of TPC and total antioxidant capacity among 15 bark extracts.
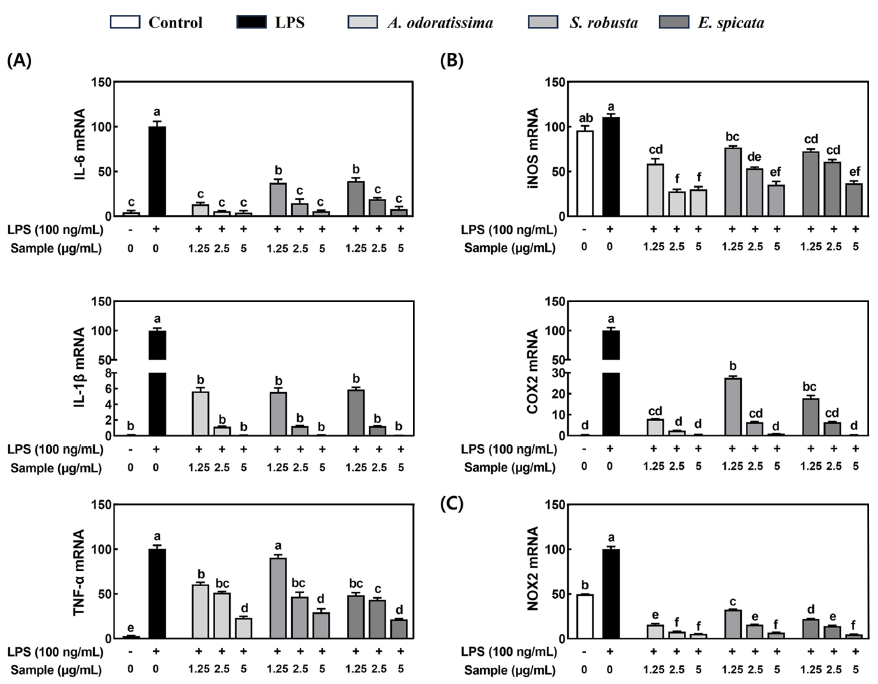
Compounds with antioxidant properties are known to have anti-inflammatory abilities (Biswas, 2016). Since three bark extracts of medicinal plants such as A. odoratissima, E. spicata, and S. robusta showed higher TPC and total antioxidant capacity than the other bark extracts, we further investigated the anti-inflammatory effects in LPS-stimulated RAW 264.7 macrophages. LPS significantly increased the mRNA expression of pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, which were significantly abolished by all three bark extracts (Fig. 1A). Also, the three bark extracts significantly reduced the mRNA expression of inflammation-inducible enzymes such as inducible NO synthase (iNOS) and cyclooxygenase-2 (COX2) in LPS-stimulated RAW 264.7 macrophages (Fig. 1B). Furthermore, the mRNA expression of NADPH oxidase 2 (NOX2), an enzyme that generates ROS, was significantly increased by LPS in RAW 264.7 macrophages while three bark extracts significantly suppressed the LPS-induced NOX2 mRNA expression (Fig. 1C).
Discussion
Phenolic compounds in medicinal plants have the ability to provide antioxidant and anti-inflammatory effects, which help to alleviate oxidative stress and inflammation at the cellular level (Tungmunnithum et al., 2018). Studies have demonstrated that Bangladeshi medicinal plants exert antioxidant capacity and inhibit pro-inflammatory cytokine secretion (Uddin et al., 2004;Yesmin et al., 2019;Islam et al., 2020;Kundu et al., 2022). While most research has focused on the inhibitory effects of leaves, fruits, or whole plant parts on oxidative stress and inflammation, it's worth noting that other components, such as bark or stems, may also contain bioactive compounds (Mahabub-Uz-Zaman et al., 2009;Hossain, 2012;Hasan et al., 2014;Bakhtiar et al., 2015;Pal & Sil, 2023). However, the effects of bark extract of Bangladeshi medicinal plant on antioxidant and anti-inflammatory functions have not been confirmed. In this study, we found that extracts derived from the bark of three medicinal plants in Bangladesh, namely A. odoratissima, E. spicata, and S. robusta, exhibited the highest total antioxidant capacity compared to 15 different types of bark extracts. Also, these three bark extracts showed anti-inflammatory properties by reducing the expression of pro-inflammatory cytokines and inflammation-inducing enzymes in LPS-stimulated RAW 264.7 macrophages.
Phenolic compounds are known to have superior antioxidant ability due to particular chemical structures, which contain conjugated double bonds and hydroxyl groups (Singla et al., 2019). In the present study, three bark extracts, A. odoratissima, E. spicata, and S. robusta exhibited the highest TPC among 15 types of bark extracts of Bangladesh medicinal plants, which was considered to have the highest phenolic compounds content formed by conjugated double bonds and hydroxyl groups. Also, three bark extracts showed the highest DPPH and ABTS radicals scavenging activity and FRAP activity among 15 types of bark extracts. The strong antioxidant properties of three bark extracts are partly due to their high phenolic compound content, which exerts a scavenging effect on ROS and interface with oxidative free radicals (Hossain et al., 2011). Furthermore, studies have demonstrated that high abundance of phenolic compounds and strong antioxidant capacity of three bark extracts are closely related to traditional uses to improve body’s immune system against ROS. The bark extract of A. odoratissima, which has been traditionally used in Bangladesh to treat diseases such as coughing, bronchitis, and diabetes, has been reported to contain flavonoids and terpenoids (Powar et al., 2020). Similarly, the bark extract of Shorea robusta, which is known for medicinal use in treating scabies and skin diseases in Bangladesh, contains major components such as flavonoids and tannin. (Singh et al., 2016;Bainsal et al., 2020). Also, phenolic derivatives such as flavonoids, triterpenes, diarylheptanoids, and aromatic acid/esters were isolated from the genus Engelhardia (Pang et al., 2018).
Multiple inflammatory triggers, such as overproduction of ROS during oxidative metabolism, have been documented to initiate the inflammatory cascade, leading to the production and release of proinflammatory cytokines (Hussain et al., 2016). Furthermore, elevated production of ROS resulting from oxidative stress can induce the oxidation of amino acids, lipid peroxidation, and oxidative damage to DNA, thereby triggering an inflammatory response (de Souza et al., 2007). In this study, the three bark extracts, including A. odoratissima, E. spicata, and S. robusta significantly suppressed the mRNA expression of inflammatory cytokines and inflammation-inducible enzymes in LPS-stimulated macrophages. Also, three bark extract exhibited the highest phenolic content with total antioxidant capacity by reducing ABTS and DPPH free radicals scavenging activity and FRAP capacity. The high antioxidant capacity of phenolic compounds can reduce oxidative stress-mediated inflammation through the inhibition of nuclear factor (NF)-κB-mediated proinflammatory cytokines (Hussain et al., 2016). Therefore, the strong antioxidant capacity and high phenolic content of the three bark extracts likely contribute to suppressing LPS-induced inflammation in RAW 264.7 macrophages by reducing oxidative stress. In particular, three bark extracts completely suppressed LPS-induced the mRNA expression of NOX2 in macrophages. NOX2 is known to regulate the production of ROS within macrophages, which stimulates the inflammatory process by secreting pro-inflammatory cytokines (Singh et al., 2016). Although this study did not evaluate the effect of three bark extracts on ROS production in macrophages, three bark extracts were observed to potentially reduce the elevated ROS production by inhibiting NOX2 gene expression in LPS-induced RAW 264.7 macrophages. Finally, since the three bark extracts exhibited strong antioxidant and anti-inflammatory abilities, it is necessary to find phenolic compounds in the three bark extracts that scavenge free radical activity and inhibit pro-inflammatory cytokines.
Conclusions
Since barks are considered potential antioxidant and anti-inflammatory resources, this study evaluated and screened three potential candidates among 15 bark extracts from medicinal plants in Bangladesh. Our findings demonstrated that among the 15 bark extracts from medicinal plants in Bangladesh, three bark extracts including A. odoratissima, E. spicata, and S. robusta have the strongest antioxidant abilities and anti-inflammatory effects in LPS-stimulated RAW 264.7 macrophages. These three bark extracts exhibited the strong total antioxidant capacity with high amount of phenolic compounds contents, which contributed to suppressing LPS-stimulated inflammation in RAW 264.7 macrophages. Constantly, three bark extracts significantly reduced LPS-induced the mRNA expression of pro-inflammatory cytokines and inflammation-inducible enzymes including IL-6, IL-1β, TNF-α, iNOS, COX2, and NOX2. Therefore, our findings highlight the substantial potential of these three bark extracts as valuable nutraceutical resources for the prevention and treatment of chronic inflammatory diseases, warranting further research on the underlying mechanisms and analysis of bioactive compounds. Furthermore, our study could offer valuable insights into utilizing global herbal resources for domestic researchers engaged in antioxidant-related research.