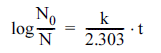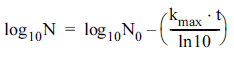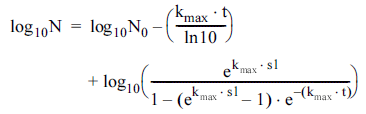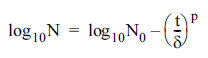Introduction
Domestic environment, especially kitchen, is a well-known source of microbial contamination and numerous foodborne illnesses have been linked to domestic infection sources (EFSA 2015; Marotta et al., 2018). Different dishwashing tools like kitchen sponges can harbor (serve as reservoir) spoilage microorganisms as well as pathogenic strains, which cause foodborne illnesses, and can also serve as vehicles of microbial transmission (Marotta et al., 2018;Sharma et al., 2009).
The levels of bacterial contamination at different sites in kitchen environments have been sufficiently investigated (Finch et al., 1978;Speirs et al.,1995). In a previous study, Gram-positive cocci have been shown to be predominant in cloths used for wiping surfaces (dishclothes, tea towels and sponges) and Gram-negative rods were found to be prevalent in the sink area (Speirs et al., 1995). In addition, Micrococcus spp. and Staphylococcus spp. were predominantly found in all of the tested kitchens and food poisoning bacteria were infrequently detected, although individual isolates of Bacillus cereus, Yersinia enterocolitica and Listeria monocytogenes were recorded (Speirs et al., 1995). In another study, an assessment on the microbiological safety of kitchen sponges, which were randomly collected from food establishments, indicated that the sponges are loaded with high levels of mesophilic aerobic bacteria, coliforms, yeast and molds, and Enterobacteriaceae (Wolde & Bacha, 2016).
The survival and spread of pathogens in the kitchen can be prevented by sponge disinfection (Sharma et al., 2009). In a previous study, microwaving (frequency of 2,450 MHz and 1.30 kW) and dishwashing treatments have been shown to be more effective in disinfecting wet kitchen sponges compared with chemical treatments (10% household bleach solution, lemon juice) (Sharma et al., 2009). In that study, following microwaving and dishwashing treatments, the counts of aerobic bacteria were decreased to <0.4 log and 1.6 log CFU/ sponge, respectively; and the counts of yeasts and molds were reduced to <0.4 log and 0.4 log CFU/sponge, respectively.
Cold plasma technology is an emerging disinfection method. Different types of non-thermal plasmas can be employed for the decontamination of processing surfaces and the surfaces of abiotic materials such as synthetic membranes and glass (Misra et al., 2011). Corona discharge is one among different approaches for plasma generation at atmospheric pressure and these discharges in air exhibit a strong bactericidal effect (Kim et al., 2015; Puligundla et al., 2018). In the present study, the inactivation effects of corona discharge plasma jet (CDPJ) against selective foodborne pathogens inoculated into different dishwashing tools were investigated.
Materials and Methods
Dishwashing tools used in the present study, namely a nonwoven dishcloth (made of 80%viscose, 20%polyester), Scotch-Brite ® sponge (made of polyester nonwoven fabric, synthetic resin and abrasive material) and Scotch-Brite ® scourer (made of nylon nonwoven fabric, synthetic resin and abrasive material), were purchased from a local grocery store.
Standard cultures of Escherichia coli O157:H7 NCTC 12079, Staphylococcus aureus KCTC 3881 and Pseudomonas putida KCCM 11210 were procured from Korean Culture Center of Microorganisms (KCCM, Seoul, Korea).
CDPJ was generated at atmospheric pressure conditions using a custom-made apparatus (Plasma Life Co. Ltd., Incheon, Korea). The plasma generator was powered by a high voltage (20 kV DC output voltage) power source, with an input current of 1.5 A and a frequency of 58 kHz, as described in earlier reports (Kim et al., 2015;Lee et al., 2017). Streamertype corona discharge in air was observed between tungsten electrodes under high voltages. In order to create plasma jet, a centrifugal air blower (Ventur Tekniska, Goteborg, Sweden) was used at a rotational (impeller) speed of 3312 rpm. Air velocity at the tip of the electrodes was 2.5 m/s.
The E. coli O157:H7 and S. aureus were cultured at 37 o C for 24 h using tryptic soy broth (BD Company, Le Pont de Claix, France) prior to use in plasma treatment experiments. And, the P. putida was cultivated in buffered peptone water (BD Company, Le Pont de Claix, France) at 25 o C for 48 h. The dishwashing tools cut into pieces of 2 × 2 cm were separately inoculated with each pathogen culture (100 μL per piece, spotted on the surfaces) and allowed to dry for an hour and then treated by CDPJ for different durations (0-5 min).
A first-order kinetic model is commonly used to explain the inactivation pattern of microorganisms upon exposure to lethal agents, as given in Eq. (1)
However, the above equation is valid for log-linear inactivation curves only. Often, microbial survival curves exhibit non-log-linear relationships. Therefore, to analyze nonlog- linear survival curves obtained following CDPJ treatment, the following models were tested using GinaFIT software (version 1.6, 2012, BioTeC, Leuven, Belgium):
Log-linear model (Bigelow & Esty, 1920):
Log-linear with shoulder model (Geeraerd et al., 2000):
Weibull model (Mafart et al., 2002):
where,
All treatments were performed in triplicate, and results are presented as mean ± standard deviation. Data were statistically analyzed using SAS statistical software package (version 9.2, SAS Institute Inc., Cary, NC, USA). The statistical significance of results was analyzed by one-way analysis of variance (ANOVA), followed by Duncan's multiple range test at α=0.05.
Results and Discussion
Treatment time-dependent inactivation of the pathogens was noted in all the tested dishwashing tools upon CDPJ treatment. On dishcloth, upon CDPJ treatment for 0-5 min, E. coli O157:H7, S. aureus and P. putida were inactivated maximally by 4.31 ± 0.21, 3.73 ± 0.18 and 3.85 ± 0.11 log CFU/cm 2 (Fig. 1). On sponge, E. coli O157:H7 and P. putida exhibited similar levels of inactivation (4.37 ± 0.21 and 3.83 ± 0.11 log CFU/ cm 2 , respectively) during the same treatment period; however, S. aureus exhibited relatively higher levels of inactivation (4.43 ± 0.22 log CFU/cm 2 ). On scourer, E. coli O157:H7 and S. aureus exhibited the highest levels of inactivation (4.56 ± 0.21 and 4.78 ± 0.22 log CFU/cm 2 , respectively) as compared to other tested materials; but P. putida exhibited an increased resistance to inactivation, only 3.50 ± 0.12 log CFU/ cm 2 reduction was observed during CDPJ treatment for 5 min.
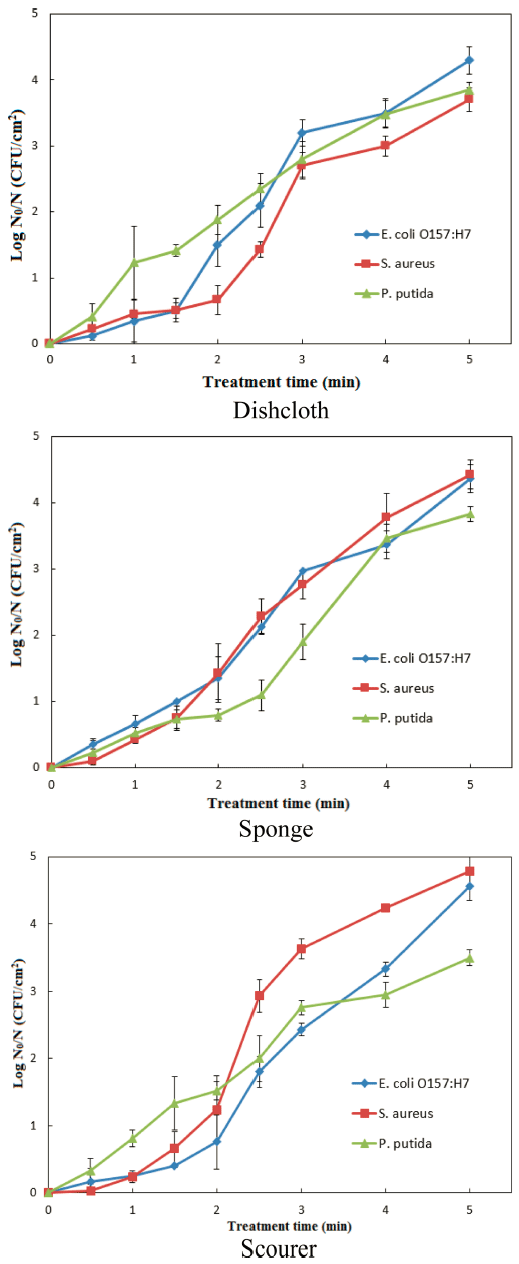
Kitchen cleaning materials have been shown to harbor large numbers of bacteria, especially Pseudomonas spp. were most commonly identified (Enriquez et al., 1997). The presence of S. aureus on kitchen cleaning materials has been reported previously (Hilton & Austin, 2000). Lee et al. (2010) have showed that a surrogate strain of E. coli O157:H7 can grow well on dishcloths and sponges. Therefore, these three types of bacteria were chosen as test contaminants in this study.
Several factors influence the efficacy of CDPJ treatment. The structural characteristics of the cleaning materials used in this study seem to play an important role in microbial inactivation. Relatively low levels of inactivation of the tested bacteria in dishcloth could be due to its micro-fibrous nature, which may provide a protective environment for the microorganisms. On the other hand, the structures of the sponge and the scourer, being more porous or cavernous in nature, appear to have allowed an increased penetration of excited plasma species, resulting in higher inactivation. Chemical composition of the tested materials might have also influenced the inactivation efficiency. From a microbiological perspective, the variation in susceptibility among bacteria seems to be species-specific, but it could also be due to complex nature of the interaction between plasma species and microorganisms hosted in different environments, as stated in a previous study (Puligundla et al., 2017).
Plasma process factors like voltage, current, frequency and field strength are known to have strong influence on microbial inactivation. Environmental relative humidity, temperature and airflow rate may influence inactivation efficacy significantly. It has been shown earlier that, at a relative humidity of 51%, maximal reductions in the viability of E. coli O157:H7, Salmonella typhimurium, and S. aureus were observed following CDPJ treatment (Kim et al., 2020). Other influencing factors include, but not limited to, density of material being treated, distance between samples and plasma emitter, direct or remote exposure, and the treatment time.
The suitability of selective models, namely log-linear, loglinear with shoulder, and Weibull, to explain the bacterial inactivation by CDPJ was tested. Most of the inactivation curves exhibited an initial lag phase (shoulders). Among the tested kinetic models, log-linear with shoulder model was fitted well to the inactivation data and therefore it was regarded as the most suitable model. This model describes the survival curves through two parameters: the inactivation rate (kmax), defined as the slope of the exponential part of the survival curve, and the shoulder length (sl), defined as dose or time before the exponential inactivation begins (Gouma et al., 2015). The suitability of the model was established based on the values of sum of squared errors (SSE), root mean squared error (RMSE), and coefficient of determination or fit index (R 2 ). As can be seen from Table 1, relatively low SSE and RMSE values and a high R 2 value for log-linear with shoulder model imply that the model is the best-fitting model compared with others. Shoulder phases are often observed in survival curves for UV-C light (Gouma et al., 2015). In a previous study, the log-linear regression plus shoulder model has been shown to provide a good fit to curves of the inactivation of Cronobacter sakazakii by UV-C light (Arroyo et al., 2012). In addition, the log-linear regression plus shoulder model was successfully used for modeling the inactivation of UV resistant strains of different pathogenic bacteria, namely E. coli, S. typhimurium, L. monocytogenes, and S. aureus, by simultaneous application of UV radiation and heat at sublethal temperatures (Gouma et al., 2015). Shoulders are related to the DNA damage and repair phenomena, according to the ‘multihit’ target theory. Up to certain UV doses, DNA repair systems can repair the damaged DNA, resulting in shoulders (López-Malo & Palou, 2005). A similar mechanism could be anticipated for shoulder phases following CDPJ treatment in this study as corona discharges generate UV radiations.
Although a pseudo-first-order kinetic model has been successfully used to describe CDPJ-induced microbial inactivation, especially in the case of seed contaminants (Puligundla et al., 2017), the log-linear with shoulder model was well suited for this study. The initial lag in microbial inactivation indicate protection offered by the hosted environment against CDPJ-induced damage up to a certain level. The log-linear with shoulder model was used to determine kinetic parameters for the inactivation of E. coli O157:H7, S. aureus, and P. putida following CDPJ treatment. As shown in Table 2, E. coli O157:H7 exhibited relatively high kmax (first order inactivation rate constant) values compared with others, indicating high susceptibility to CDPJinduced inactivation. The kmax values observed for E. coli O157:H7 on dishcloth, sponge, and scourer were 2.42 ± 0.28, 2.71 ± 0.16, and 2.81 ± 0.16 min−1 , respectively.
| Dishwashing tool | E. coli O157:H7 | S. aureus | P. putida |
|---|---|---|---|
| Dishcloth | 2.42 ± 0.28b | 2.22 ± 0.26 b | 1.99 0.11ab |
| Sponge | 2.71 ± 0.16a | 2.36 ± 0.16a | 2.03 ± 0.44a |
| Scourer | 2.81 ± 0.16a | 2.45 ± 0.39a | 1.76 ± 0.20b |
Summary
Dishwashing tools can be decontaminated effectively by CDPJ treatment. On the surfaces of the tested dishwashing tools, E. coli O157:H7 were inactivated in the range of 4.30 - 4.56 log CFU/cm 2 ; and S. aureus and P. putida were inactivated in the ranges of 3.71-4.78 and 3.50-3.83 log CFU/ cm 2 , respectively. The kinetics of the inactivation indicated that the log-linear with shoulder model was the most suitable to explain bacterial inactivation pattern; and the rates of decontamination were decreased in the order E. coli O157:H7 > S. aureus > P. putida.