Introduction
Fresh-cut produce which provides a healthy diet of essential nutrients and minerals offers convenience to consumers since it can be consumed right away. These factors have satisfied the people’s needs of preferring a healthy diet with less amount of effort. This being so, the demand for fresh-cut produce has increased recently throughout the years (Ghidelli & Pérez- Gago, 2018). High demand in fresh-cut produce naturally lead to incidents of foodborne outbreaks (Carstens et al., 2019;Machado-Moreira et al., 2019). According to The Centers for Disease Control and Prevention (CDC) during 1995 to 2005, they reported that fresh produce associated foodborne disease outbreaks have increased in number throughout the world (Johnston et al., 2005). Moreover, Carstens et al. (2019) reported that a total of 85 outbreaks occurred from 2010 to 2017, in multiple states of USA, with a confirmed etiology, which was related to fresh produce. To be specific, Capsicum sp. have been involved in several incidents. Alwi & Ali (2014) reported that Capsicum sp. are more vulnerable to bacterial contaminations and mentioned that several studies of bell peppers have reported to be contaminated with fecal coliforms. Ginger is also another product of concern. Kim et al. (2008) reported that total aerobic bacteria and coliform bacteria of ginger were 4.4 log CFU/g and 5.4 log CFU/g, respectively. Minced ginger is also a concern because they may occur problems such as browning, unpleasant smell, dripping, and poor quality due to the proliferation of microorganisms (Choi et al., 2002). These studies indicate that bell pepper and ginger are contaminated with high amount of bacteria.
Accordingly, many food industries are currently trying their best to find a proper sanitizing method for fresh-cut products. In the food industry, there are different kinds of chemical, biological and physical sanitizing methods but chlorine is one of the most commonly used sanitizer due to its convenience and inexpensiveness (Meireles et al., 2016). However, safety concerns have been raised about the by-products of chlorine such as trihalomethanes and alternatives have been searched (Parish et al., 2003).
Intense pulsed light (IPL), which is a non-thermal technique seems to be a good alternative for chlorine. IPL, is known for its strong bactericidal effect on different food surfaces by emitting intense short pulses and high frequency from its wide broad spectrum. IPL spectrum includes wavelength from ultraviolet to the near infrared region (200 to 1,100 mn) and has been reported to be especially lethal in UV-C light region (Barbosa-Canovas et al., 2000;Gomez-Lopez et al., 2007). In particular, IPL does not leave any residue while quickly inactivating microorganisms, promising safe food products to the customers (Hwang et al., 2019).
Escherichia coli O157:H7 has been reported to be one of the most common pathogen related to fresh-cut produce outbreaks in several studies (Olsen et al., 2000;Buck et al., 2003;Roth et al., 2018). Escherichia coli O157:H7 is one of the strains in the Enterohemorrhagic E. coli group and the remaining bacteria cause a fatal disease outbreak once the food contaminated with E.coli O157:H7 is consumed. They cause a serious damage in the intestine or other organs due to the mass amount of toxin they produce (Harris et al., 2003). Numerous studies have reported outbreaks related to E.coli O157:H7 associated fresh produce such as spinach (Sep, 2006;Grant et al., 2008), lettuce (Hilborn et al., 1999) and from many other food stuffs (Rangel et al., 2005).
The objective of this study, therefore, was to evaluate the microbial quality of fresh-cut bell pepper (Capsicum annuum L) and ginger (Zingiber officinale), and the bactericidal effect of IPL on E.coli ATCC 25922, as a surrogate for E. coli O157: H7, inoculated in the fresh-cut samples by varying the voltages (1,200, 1,600, 2,000, and 2,400 V) and treatment time (1, 2, 3, 5 and 7 min). Through the study, we tried to evaluate the prevalence rate of the microorganisms and seek the possibility of the practical application of IPL to fresh-cut bell pepper and ginger.
Materials and Methods
Microbiological analysis were performed according to the AOAC Official Method (AOAC International, 2002) and the Korea Food Code (MFDS, 2019) with some modifications. Twenty-five grams of each sample was evenly selected, transferred into a sterile stomacher bag and homogenized using a stomacher (Bag mixer 400CC, Interscience, Saint- Nom-La-Breteche, France) with 225 mL of sterile buffered peptone water (BPW, Difco, Sparks, MD, USA) to obtain a homogenate. Aerobic mesophilic plate count for produce samples were tested using 3M Aerobic Count Plates (3M, St. Paul, MN, USA) in accordance with the AOAC Official Method 990.12. The BPW homogenate was serially diluted using 10-fold dilution method with 9 ml of BPW and 1 mL of each dilution was plated in duplicate. The colonies were then estimated after incubation at 36 °C for 48 h. For psychrophilic plate count, 0.1 ml of each BPW dilution was spread on the PCA plates in duplicate and was incubated at 6 °C for 5-7 days.
Strains of Escherichia coli ATCC 25922 (KCCM 11234) was in a frozen form containing 20% (v/v) glycerol. In this study, E.coli ATTC 25922 was used as a surrogate for E.coli O157:H7 referring to a study conducted by Sauer & Moraru (2009). For pre-culture, a loop of the frozen stock was inoculated into 10 mL of tryptic soy broth (TSB, Difco) and was incubated in 37 °C for 24 h in a 180 rpm shaking incubator. Afterwards, 1 mL of the grown culture was transferred to 100 ml of TSB and was incubated at 37 °C for 5 h 180 rpm in a shaking incubator until it reached the exponential phase. The inocula were then harvested by centrifuge for 10 min at 4,000×g. Subsequently, the supernatant was discarded and the pellet was refrigerated after it was washed again in sterile 0.85% NaCl solution.
Bell pepper and ginger was purchased from a local supermarket. After purchase, they were kept in a refrigerator and were used within 24 h for treatment. Then they were washed with tap water and was cut into pieces of 2.5 × 2.5 × 0.5 cm (wide × length × height) with a sterilized knife. Each of the samples were then placed into an empty petri-dish and was spot inoculated with 100 μL of the prepared E. coli inoculum (106 CFU/mL) in the upper surface with a sterile micropipette. The inoculated samples were dried for 30 min to 1 h on room temperature (~20 °C) inside the laminar-flow hood before treatment.
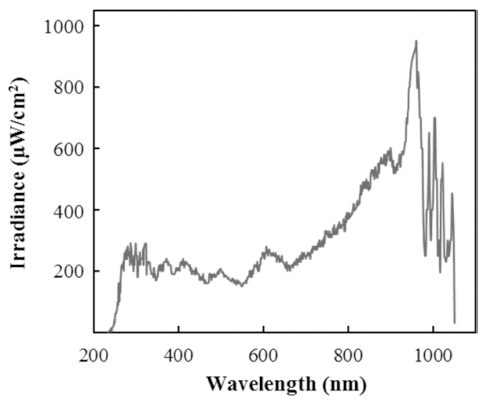
IPL treatment was done using a device designed in the laboratory. The device composed a power unit and a treatment chamber with a xenon lamp (NL9553 lamp, XAP series, Heraeus Noblelight, Cambridge, UK) on the top. Fig. 1. shows the spectral distribution of IPL generated by xenon lamp (NL9553) and it was measured by a spectroradiometer (ILT950, International Light Technologies, Peabody, MA, USA). Also, there is a cooling system which, distilled water flows through the lamp, preventing overheating during the treatments. For the treatment, the distance between the sample and the lamp was 8 cm, had a frequency of 2 Hz and the pulse duty was 0.5 ms. These conditions did not change during the study and only the voltage and treatment time varied. Samples were treated with 4 different voltages (1,200, 1,600, 2,000, and 2,400 V) and 5 different treatment time (1, 2, 3, 5, and 7 min) respectively.
For Escherichia coli counts, 3M Coliform/E. coli Count Plates (3M) were used in accordance with the AOAC Official Method 991.14. Each of the treated sample was homogenized with 1:9 ratio of sterile 0.85% saline for 2 min using a stomacher (Bag mixer 400CC). Then, the homogenate was serially diluted using a 10-fold dilution method with 9 mL of 0.85% saline and 1 mL of each dilution was plated in duplicate. Petrifilms were then incubated at 37 °C for 24-48 h for further calculation. The calculation was done by following the instructions. The microbial reduction were calculated in log as the number of survived microbial counts after the treatment (N) to the initial microbial counts (N0).
Results and Discussion
In order to evaluate the microbial quality of fresh-cut bell pepper and ginger, total viable counts of mesophilic, psychrophilic bacteria and yeasts and molds of bell pepper and ginger were studied and shown in Table 1-3. For mesophilic bacteria, bell pepper ranged from 5.23 to 7.23 log CFU/g with mean counts of 6.64 log CFU/g and ginger ranged from 2.00 log to 6.95 log CFU/g with mean of 6.35 log CFU/g (Table 1). For bell pepper, every samples showed high microbial counts whereas some samples of ginger showed relatively lower counts such as 2.00 log CFU/g. Yu et al. (2011) surveyed 320 non fresh-cut paprika and mean counts of total aerobic bacteria were reported to be 2.3±0.3 log CFU/g. Yu’s study suggests that paprika contained low amount of aerobic bacteria. Alexopoulos et al. (2013) also reported that the surface microflora on bell pepper were approximately 3 to 4 log CFU/g for aerobic mesophilic bacteria and yeasts and molds. The high level of viable counts of bell pepper in this study, however, seems to be due to the characteristics of the fresh-cut product that was purchased. When the product was purchased, it was already cut into pieces (in a cut form) which could have made it vulnerable to microorganisms. For ginger, Lee et al. (2018) analyzed 24 samples of ginger while evaluating the microbiological contamination of kimchi and its ingredients. They reported that among the ingredients, ginger had the highest count of 8.8 log CFU/g in total aerobic bacteria. A similar study conducted by Kim et al. (2010) also showed results of ginger having the highest viable counts of aerobic counts which had counts over 7 log CFU/g. In case of psychrophilic bacteria, bell pepper ranged from 4.82 to 7.16 log CFU/g with mean counts of 6.75 log CFU/g and ginger ranged from 1.40 to 6.23 log CFU/g with mean of 5.63 log CFU/g (Table 2). Even though some samples showed low microbial results, mean counts of both bell pepper and ginger exceeded 5 log CFU/g and this showed that the contamination level of theses samples were high. For yeasts and molds, bell pepper resulted in range from 3.77 to 4.90 log CFU/g with mean counts of 4.68 log CFU/g and ginger ranged from 1.80 to 5.20 log CFU/g with mean of 4.57 log CFU/g (Table 3). Kim et al. (2009) have reported that ginger is prone to damage during the shipment process because it has a humid and tender surfaces, resulting in mold-induced decay. After the manufacturing procedure, ginger and bell pepper are likely to be vulnerable to bacteria or yeasts and mold due to their exposed surfaces. Moreover, the contamination level of YM in fresh-cut foods should be concerned and monitored to ensure acceptable levels because several foodborne molds and yeasts in foodstuffs can produce mycotoxins (Tournas et al., 2001). Consequently, the results indicate that fresh-cut bell pepper and ginger are generally contaminated with high levels of aerobic bacteria and YM and therefore, thorough hygiene control seems to be necessary to improve the quality of these product.
| Sample | Total aerobic mesophilic bacteria | ||
|---|---|---|---|
| Minimum | Maximum | Mean1) | |
| Bell pepper (n=5) | 5.23 | 7.23 | 6.64±0.81 |
| Ginger (n=5) | 2.00 | 6.95 | 6.35±1.96 |
| Sample | Total aerobic psychrophilic bacteria | ||
|---|---|---|---|
| Minimum | Maximum | Mean1) | |
| Bell pepper (n=5) | 4.82 | 7.16 | 6.75±1.13 |
| Ginger (n=5) | 1.40 | 6.23 | 5.63±1.89 |
| Sample | Yeasts and molds | ||
|---|---|---|---|
| Minimum | Maximum | Mean1) | |
| Bell pepper (n=5) | 3.77 | 4.9 | 4.68±0.43 |
| Ginger (n=5) | 1.80 | 5.2 | 4.57±1.25 |
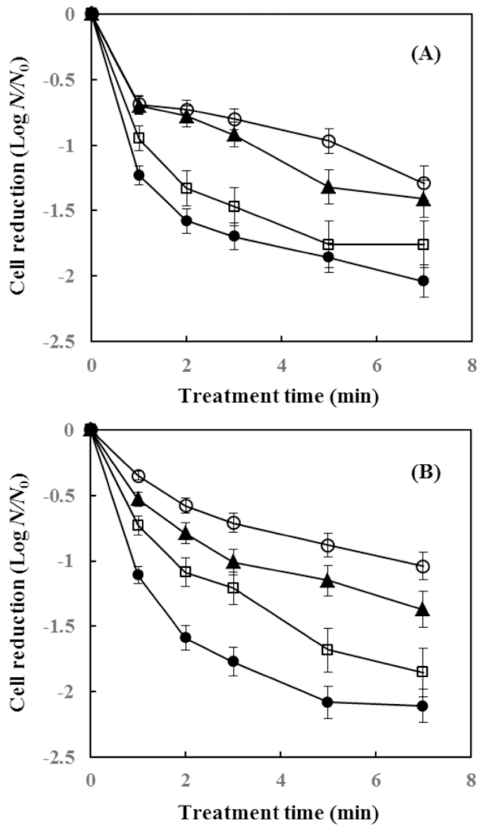
The Inactivation effect of IPL treatment on E.coli ATCC 25922 inoculated in bell pepper and ginger is shown in Fig. 2. When the treatment time was set for 1 min, 0.69, 0.7, 0.95, and 1.23 log reduction was achieved in bell pepper with treatment voltage of 1,200, 1,600, 2,000, and 2,400 V, respectively. Moreover, for 7 min, 1.29, 1.41, 1.76, and 2.04 log reduction was achieved as the voltage increased. In case of ginger, 0.35, 0.53, 0.73, and 1.11 log reduction was achieved as the voltage increased through 1,200 to 2,400 V for 1 min of IPL treatment, and for 7 min treatment, 1.04, 1.37, 1.85, and 2.11 log was reduced as the voltage increased. Although the reduction rate varied, the bactericidal effect of IPL on E.coli ATCC 25922 increased as treatment time and voltage increased. In the beginning, the reduction curve of E.coli ATCC 25922 did not show any shoulder line and as the treatment time increased, the tailing phenomenon was observed. This phenomenon was also observed in the study of Kim & Shin (2015) where they inactivated E.coli O157:H7 by IPL. Also, Izquier & Gómez- López (2011) reported the same results. Gomez-Lopez et al. (2007) and Yaun et al. (2003) explained the tailing effect in the inactivation curve as the shadowing effect, multi-hit phenomena, lack of homogeneous population, presence of suspended solids, the use of different strains that may affect their susceptibility to UV-C and varying abilities of cells to repair DNA mutations. There were also other studies of bell peppers treated with IPL. Gomez-Lopez et al. (2005) reported 0.37 and 0.56 log reduction in bell pepper with treatment time of 45 sec and 180 sec equivalent to 675 and 2700 pulses, respectively. Also, Hong et al. (2013) reported 1 log reduction of aerobic count with treatment conditions of 1,000 V, 5 pps (pulse per second) for 10 min. In the study of Luksiene et al. (2012), sweet peppers were illuminated with treatment conditions of 1,400 V, 1,000 pulses and UV light dose 5.4 J/cm2 , and 1.3 and 1.8 log was reduced for mesophiles and B.cereus, respectively. Most such studies, however, focused on vegetables such as bell pepper, and studies regarding the effect of IPL treatment on ginger are lacking. Thus, for the commercial applicability of IPL, further studies for decontamination of ginger are required.
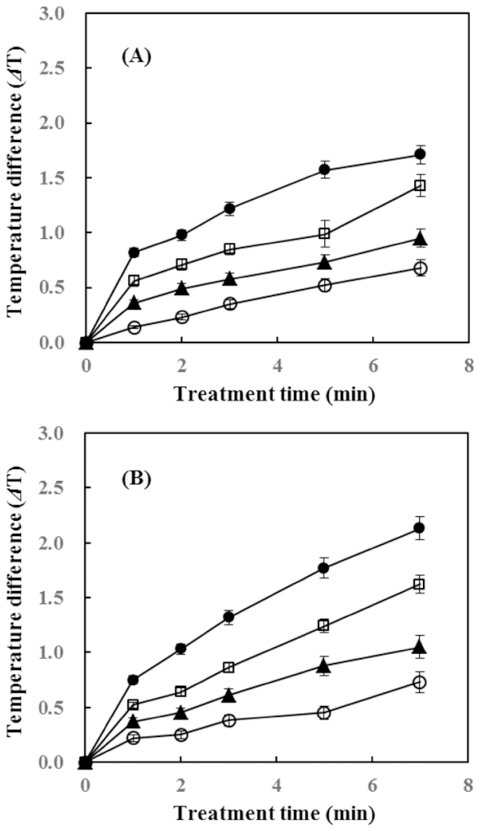
The temperature change after IPL treatment for bell pepper and ginger at each voltage (1,200, 1,600, 2,000, and 2,400 V) is shown in Fig. 3. Temperature change in a particular food sample can affect the nutritional and organoleptic properties such as loss of nutrients, change in color and flavor etc. (Raso & Barbosa-Cánovas, 2003;Kim & Shin, 2014). For bell pepper, the temperature increased only 1.71 °C at the maximum IPL treatment condition (2,400 V, 7 min) and ginger showed a temperature rise of 2.13 °C under the same condition of IPL. Kim & Shin (2014) conducted a study of the temperature increase of dried laver after IPL, and 1.9 °C increased when it was treated with conditions of 1,000 V, 5 pulse per second for 10 min with distance of 7.9 cm. The result of the study indicated that the temperature rise was marginal that it did not affect the quality of the product. However, there were also studies that showed high increase in temperature. Ignat et al. (2014) reported that apple slices treated with 0, 17.5, 157.5 kJ/ m 2 of pulsed light had temperature increased from 5.9±0.1 °C to 8.7±0.4 °C and 25.8±0.3 °C respectively. Moreover, Izquier & Gómez-López (2011) studied the temperature increase of lettuce, cabbage and carrot, and the highest increase in temperature was 9 °C in cabbage when it was treated with 12 PL fluences (J/cm2 ). Differences of temperature increase presented in various studies is thought to be due to the effect of cooling system contained in the IPL treatment device. In this study, the device used for IPL treatment has a cooling system which, distilled water flows through the lamp, preventing overheating during the treatments. Consequently, in this study, the results suggest that IPL treatment seems to guarantee the quality of the food properties due to the low temperature rise in both bell pepper and ginger during IPL treatment.
Conclusion
The purpose of this study was to evaluate the microbiological quality of fresh-cut bell pepper and ginger and the bactericidal effect of IPL on E. coli ATTC 25922 inoculated in the fresh-cut samples. The contamination levels of mesophilic bacteria, psychrophilic bacteria and yeast and mold (YM) for bell pepper and ginger were 6.64±0.81 and 6.35±1.96 log CFU/g, 6.75±1.13 and 5.63±1.89 log CFU/g, and 4.68±0.43 and 4.57±1.25 log CFU/g, respectively. IPL treatment at 2,400 V for 7 min produced 2.04 and 2.11 log reduction of E. coli ATCC 25922 for bell pepper and ginger, respectively, with a negligible temperature rise (< 2.2 °C). Therefore, the sanitizing effect of IPL seems to be quite promising in inactivating E. coli ATCC 25922 on bell pepper and ginger. Besides, during IPL treatment, low temperature rise in the samples promises the quality from samples changing inappropriately. However, for the application of IPL on a commercial scale, future studies should investigate the effects of various treatment conditions and limiting factors for IPL on various fresh-cut foodstuffs in order to seek the optimal result.