Introduction
The mud loach (Misgurnus mizolepis) is a small, longcylindrical freshwater fish belonging to the Cypriniformes (Carreon et al., 1976). It is widely distributed in rice fields and rivers containing mud. About 860 tons of mud loaches were grown in Korea in 2015 as a freshwater aquaculture species (MOF, 2016). The mud loach has also been widely used as an experimental model fish due to the success of induced multiple spawning (Nam, 2006).
The mucus covering the mud loach is composed of mucus cells and epidermal tissue and protects the mud loach against outside attacks. Mucus is a multifunctional substance with very diverse roles not only in protection, but also in breeding, excretion, and communication (Reverter et al., 2018;Wang et al., 2014). Mucus contains water, salt, immunoglobulins, secretory proteins, and mucin (a glycoprotein that is the most abundant macromolecule in mucus). Mucin is composed of 75% carbohydrates and 25% amino acids (N-acetylgalactosamine, serine, and threonine), and the N-acetylgalactosamine molecules are linked to the amino acid residues by O-glycosidic bonds (Berry et al., 2013;Jeong, 2008). The cross-linking of mucin molecules creates a three-dimensional structure in water, resulting in a highly viscous fluid and a comblike structure (Bansil et al., 1995;Wang et al., 2015). However, the existing data about mud loach mucin are insufficient.
Therefore, in this study, the production process of mud loach mucin was standardized according to optimum mucin yield. Furthermore, the physicochemical and functional characteristics of mud loach mucin were measured.
Materials and Methods
Mucin was obtained from Mirage Food (Incheon, Korea). Ethanol (60-100%) was used as the extraction solvent for Misgurnus mizolepis mucin. The mucin extraction process is shown in Fig. 1 (Sanae et al., 1982). Extracted samples were stored at -18°C. Distilled water was added to the mucin fraction (MF) to create an aqueous mucin solution, and the mucin content of the MF was measured with a Mucin Assay Kit (Cat. No. CSR-FFA-MU-K01E, Cosmo Bio Co., Ltd., Tokyo, Japan).
The storage modulus (G’) and loss modulus (G”) of mucin were investigated with a rheometer (RheoStrass RS1, Thermo Haake, Karlsruhe, Germany) at constant frequency (1 Hz) and stress (0.1 Pa). A plate-plate system (diameter: 6 cm, gap: 250 μm) and a temperature sweep (20-80°C) were used, and data were analyzed with RheoWin Data Manager (version 4.63, 0002).
The thermal properties of mucin were investigated with a differential scanning calorimeter (DSC 4000, Perkin Elmer, Waltham, MA, USA) with a sealed empty pan as the reference. The mucin suspension was sealed in a stainless-steel pan and scanned from 20 to 220°C at a heating rate of 5°C/min. The endothermic curve, maximum peak temperature (Tp), completion temperature (Tc), and melting enthalpy (ΔH) were obtained (Choi et al., 2011).
Bacillus cereus (gram-positive bacteria) and Escherichia coli O157: H7 (gram-negative bacteria) were cultivated in liquid media (Brain Heart Infusion; BHI) and Luria-Bertani (LB), respectively, containing 0.5% (w/v) mucin. The absorbance was measured at 600 nm every 2 h on a spectrophotometer (Beckman Coulter DU 730, Beckman Co., Brea, CA, USA). In the same manner, the absorbance values of the media were measured (control), and the differences in optical density (O.D.) values were used to determine antimicrobial activity (An et al., 1997).
The antioxidant activity of mucin was measured with a vitamin C equivalent antioxidant capacity (VCEAC) assay using 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Park et al., 2009). The absorbance reduction of the sample (mg/100 mL) was calculated as vitamin C equivalents in mg/100 g. For comparison, 50 ppm L-cysteine was used, and the results were calculated with the VCEAC equation (Kim & Lee, 2004).
VCEAC (mg/100 mL) = (ΔAbs - a)/b
(a: y-intercept of the vitamin C standard curve, b: slope of the vitamin C standard curve, △Abs: difference between the absorbance of the control and the sample)
The elastase inhibitory activity of mucin was measured and calculated according to James's method (Cho & Choi, 2010). Epigallocatechin gallate (EGCG) was used as a positive control, and its absorbance was measured at 250 nm (Kim & Lee, 2004).
Elastase inhibitory activity (%) = (1-B/A) × 100
(A: elastase activity without the sample, B: elastase activity with the sample)
The MMP-1 and MMP-2 activity inhibition by UVA irradiation assay was modified by the method of Dunsmore et al. (1996). Human fibroblast cell line, HS68 was irradiated with UVA at 120 mJ/cm2, and cell was cultured for 24 h after adding mucin. The culture was incubated overnight at 4 °C in a 96-well plate. Antibody was reacted with anti-MMP-1 and MMP-2 (R&D systems, Minneapolis, MN, USA) to measure the absorbance at 405 nm.
The effect of mucin on the production of hyaluronic acid was investigated in HaCaT cells. Cells were incubated for 24 h in a CO2 incubator at 37°C and subjected to UVA irradiation at 5 J/cm2. A hyaluronic acid-ELISA kit (Corgenix Inc., Colorado, USA) was used (Park & Shim, 2016).
Results and Discussion
Ethanol was used to extract mucin from mud loaches. As the ethanol concentration increased from 60 to 90%, more mucin was extracted (from 97.45 to 385.69 μg mucin/kg sample). However, there was a rapid decrease in yield when 100% ethanol was used (105.77 μg mucin/kg sample). Thus, the mucin extraction yield was highest with 90% ethanol, so this ethanol concentration was used to extract mucin from mud loaches in all extraction processes.
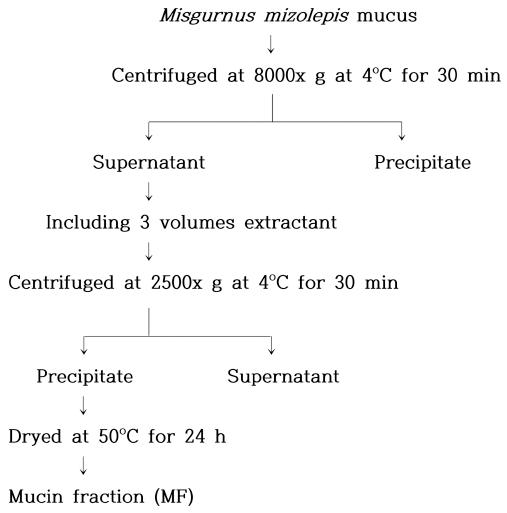
The dynamic viscoelasticity values of the mucin solutions are shown in Fig. 2(a). The initial storage modulus values (G’) were 1.205, 14.13, 19.29, and 29.46 Pa at 0.5, 1, 2, and 3% mucin concentrations, respectively. After heating, G’ increased to 1.32, 15.26, 24.75, and 33.99 Pa, respectively. This indicates that G’ is greatly influenced by mucin concentration during the heating process. At high concentrations, mucin molecules become entangled, form non-covalent bonds, and temporarily possess gel-like properties (Wang et al., 2015). Therefore, it appears that mucin has more elastic properties at higher concentrations. All of the tan δ values were less than 0.5 (Fig. 2(b)), indicating that the elastic property of mucin dominated the viscous property. Mucoproteins absorb elastic energy during heating, and the viscoelastic structure is deformed, resulting in a flowing form (Zhang et al., 2016).
The thermal properties of mucin were investigated on a DSC (Table 1). The higher was the concentration of mucin, the lower was the denaturation starting temperature and the larger was the denaturation enthalpy (ΔH). In addition, mucin was denatured only at temperatures greater than 100°C, indicating that it is a thermostable glycoprotein. In general, glycoproteins differ from pure proteins and have diverse three-dimensional structures, such as linear and branched structures (Bansil et al., 1995). Mucin also has numerous linkages connected to its polypeptide backbone. Thus, as the concentration increased, ΔH of the protein increased. Compared to dynamic viscoelasticity measurement, more concentration of mucin was needed to detect the thermal property using DSC. Higher concentration of mucin was necessary to determine the higher melting temperatures around 150-160°C.
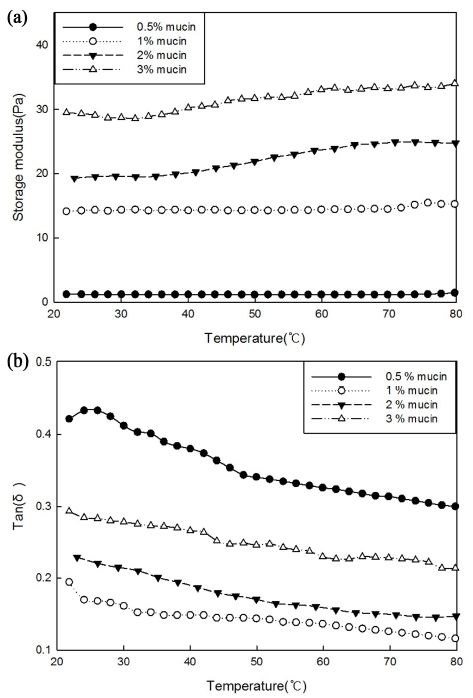
Mucin was added to the culture media of bacteria, and its microbial inhibitory effects were determined from the O.D. differences between the control media and the mucincontaining media (Fig. 3). This is the same result that the snail's mucin has antibacterial properties. The O.D. of the control medium was 0.011 for Escherichia coli O157: H7 and 0.051 for Bacillus cereus, and each was stable over time. In E. coli (Fig. 3(a)), the difference in O.D. values between control and mucin-containing media gradually increased from 0.344 after 1 h to 0.739 after 3 h, 0.831 after 5 h, and 0.942 after 7 h. Thus, the inhibitory effect of mucin against E. coli increased over time. In Bacillus (Fig. 3(b)), the interval of the graph increased to 0.598 by 3 h but decreased thereafter. This indicates that mucin has better antimicrobial activity in E. coli than in Bacillus.
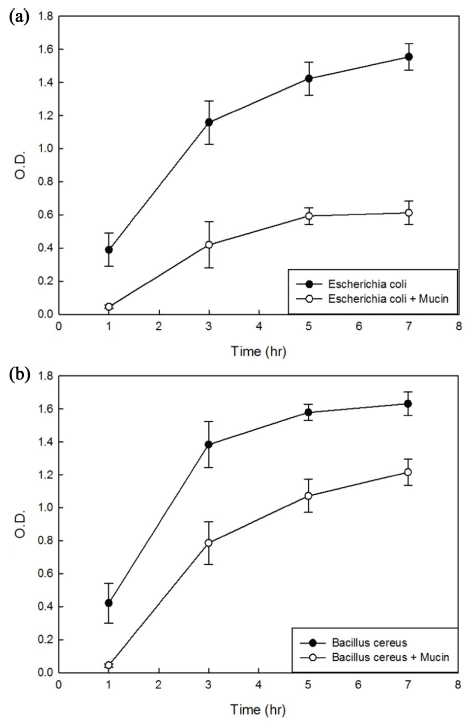
As antioxidant levels increase, the absorbance of the ABTS radical at 734 nm increases. The vitamin C standard curve exhibiting the reduction in absorbance at 734 nm was linear and was used to calculate VCEAC (Fig. 4). Mucin was measured to have 93.53 mg VCE/100 g. The antioxidant activity of the effective antioxidant L-cysteine was determined for comparison and was found to be 140.35 mg VCE/100 g. Thus, mucin had about 66.64% of the antioxidant activity of L-cysteine.
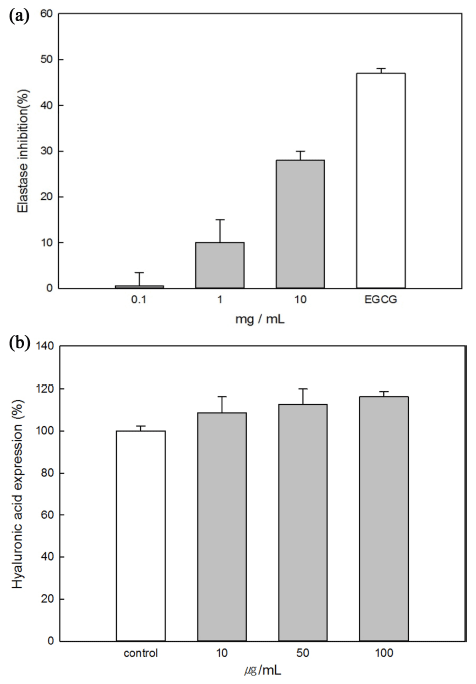
Elastase is involved in degradation of elastin and is a nonspecific hydrolytic enzyme capable of degrading collagen. It is activated in abnormal tissues, where it directly causes tissue destruction (loss of elasticity and skin wrinkles). Therefore, elastase inhibitors have the effect of improving skin wrinkles (Naoko et al., 2001). The inhibitory effect of mucin on elastase was investigated by an in vitro method. Epigallocatechin gallate (EGCG, 1 mg/mL), a well-known elastase inhibitor, was used as a positive control (Kim et al., 2004). As the mucin concentration increased, the elastase activity decreased, and the 10 mg/mL mucin sample had the highest elastase inhibitory activity (Fig. 6(a)). Mucin had about 60% of the elastase inhibitory activity of EGCG (47%).
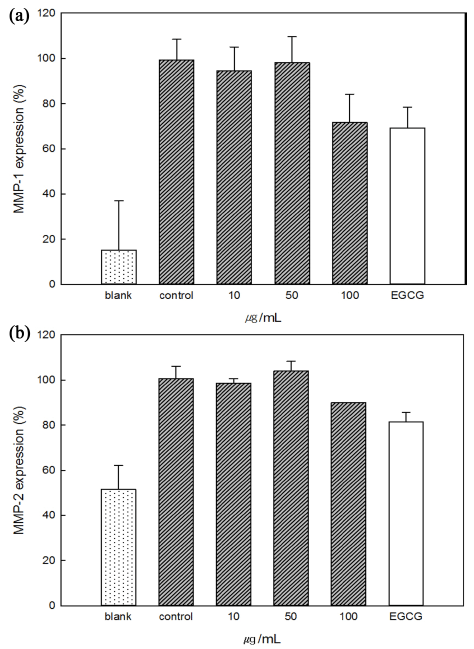
Collagen, accounting for about 90% of the dermal layer, gives strength and tension to the skin and protects the skin from external stimuli. Thus, collagen reduction in the skin is closely related to skin aging (Huang et al., 1997). Mucin showed inhibitory effects on MMP-1 and MMP-2 So the antiaging effect was confirmed. The expression of MMP-1 and MMP-2 was induced by irradiation with ultraviolet light (Control, 100%), although the expression of MMP-1 and MMP-2 was small before UV irradiation (Blank). In the case of MMP-1 shown in Fig. 5(a), MMP-1 expression (%) in all samples was lower than control and 71% in 100 μg/mL mucin sample. Fig. 5(b), the expression of 100 μg/mL mucin was 90%, which was statistically significant with 81% of EGCG. These results show that mucin inhibits enzymes that promote changes in skin wrinkles and elasticity due to ultraviolet rays or aging, and proved its efficacy as a material for skin health.
Hyaluronic acid is associated with skin moisturization, and its expression in cells is reduced by ultraviolet light exposure and aging. The effect of mucin on hyaluronic acid secretion was measured in HaCaT human keratinocytes (Fig. 6(b)). As the concentration of mucin increased, the secretion of hyaluronic acid increased, and the 100 μg/mL mucin sample had the greatest effect (116.25%). Therefore, mucin seems to be effective in moisturizing the skin (Hasova et al., 2011).
Conclusion
Experiments were conducted to optimize the extraction process of mucin in Misgurnus mizolepis epidermal mucus and to investigate its physicochemical properties and functionality. The optimum extraction conditions were 90% ethanol and the amount of mucin present was 385.69 ± 7.49 μg mucin/kg Sample. To investigate the physicochemical properties of the extracts, the thermal properties were determined using DSC and the dynamic properties were determined by measuring the viscoelasticity. The antioxidant capacity of mucins was determined by ABTS method and the antimicrobial activity of Escherichia coli and Bacillus cereus was determined by the optical density value difference. Elastase, MMP-1&MMP-2 inhibitory activity and hyaluronic acid expression ability was measured and it is indicating that mucin has moisturizing and skin protection ability. This suggests that various uses of mucin in Misgurnus mizolepis epidermal mucus may be facilitated in food and non-food industries.