Introduction
The incomplete pyrolysis or combustion of organic materials (e.g., coal, oil, gas, wood and garbage) at temperatures between 300 and 600°C results in the formation of benzo[α]pyrene (BaP), a carcinogenic polycyclic aromatic hydrocarbon. The presence of BaP is ubiquitous; ranging from coal tar to tobacco smoke to heat-treated foods. In foods, BaP is induced by heat treatments such as grilling or roasting, and therefore its formation is commonly observed in smoked/grilled meats and roasted seeds. The formation of BaP during roasting of sesame seeds has been shown in earlier studies (Cheng et al., 2015;Lee et al., 2019). The International Agency for Research on Cancer has categorized BaP as a Group 1 carcinogen; BaPinduced tumorigenesis in experimental animals has been well established (IARC, 2012). In addition, the US Environmental Protection Agency has classified as priority pollutant due to its acute toxicity, carcinogenicity and known or suspected teratogenicity (Kot-Wasik et al., 2004). BaP toxicity is mediated by oxidative metabolism to reactive intermediates that interact with macromolecules leading to alterations in target cell structure and function (Miller & Ramos, 2001). BaP is metabolized in cells by cytochrome P450 to BaP diolepoxides or BPDE that bind to DNA covalently, resulting in the formation of DNA adducts and eventually cancer initiation (Fu & Xia, 2016).
Due to its ubiquitous distribution and toxicity, BaP detoxification in foods or the remediation of BaP-contaminated sites has received considerable attention. However, due to its recalcitrant nature, BaP degradation cannot be achieved easily. Different methods such as volatilization, photo-oxidation, chemical oxidation, bioaccumulation, adsorption on sediment particles, microbiological transformation and degradation, etc., have been used for the degradation and elimination of PAHs including BaP from the environment (Wilson & Jones, 1993). But, conventional oxidants (e.g., Cl2, ClO2 and KMnO4) suffer from inherent disadvantages such as the inability to produce highly reactive species, low reaction rate, and more specific with regard to the types of organic molecules that can be oxidized. In addition, biodegradability of PAHs is low. Hence, there has been growing interest in the use of advanced oxidation processes (AOPs), which utilize oxidants such as ozone (O3) and Fenton's reagent, for PAHs degradation. Electrochemically generated O3 has been shown to be effective in degrading and detoxifying BaP (Ottinger et al., 1999). In another study, in the remediation of PAH (including BaP) from spiked and coal tar-contaminated soils, combined ozonation/ biodegradation treatments have been shown to be effective (Nam & Kukor, 2000). The effectiveness of Fenton’s reagent (a solution of hydrogen peroxide with ferrous iron as a catalyst) for the degradation of BaP in water matrices has been shown (Homem et al., 2009).
Non-thermal plasma (NTP) or cold plasma represents one of the most promising sources of oxidants. NTPs can generate different types of reactive oxygen species (ROS) (e.g., hydroxyl (HO•), hydroperoxyl (HO2•), ozone (O3)) and reactive nitrogen species (RNS) (e.g., nitric oxide (NO), peroxynitrite (ONOO−), nitrite (NO2−), nitrate (NO3−)), which have a higher oxidation potential to react with organic molecules (Attri et al., 2016). Effective degradation of organic dyes, namely methylene blue, methyl orange and Congo red, following treatments with two needle-type atmospheric pressure plasma jets (APPJs), an indirect (ID-APPJ) and a direct (D-APPJ), has been shown (Attri et al., 2016). Corona plasma discharges are known to produce reactive species such as electrons, oxygen ions and excited neutrals (Choi et al., 2017). Hence, in the present study, corona discharge plasma jet (CDPJ) generated using air under atmospheric pressure conditions was used to degrade BaP on glass slides and modelled its degradation kinetics. In addition, our study investigated CDPJ degradation of BaP in food commodities, namely roasted sesame and perilla seeds and their oils.
Materials and Methods
Benzo[α]pyrene having ≥96% purity (HPLC grade) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). LC-grade water and HPLC-grade acetonitrile were obtained from J. T. Baker (Phillipsburg, NJ, USA) and Duksan Pure Chemical Co., Ltd. (Ansan, Korea), respectively. All other chemicals used were of analytical grade quality.
Food commodities used in this study, namely sesame and perilla seeds, were procured from a local store in Yongin-si, Gyeonggi-do, Korea.
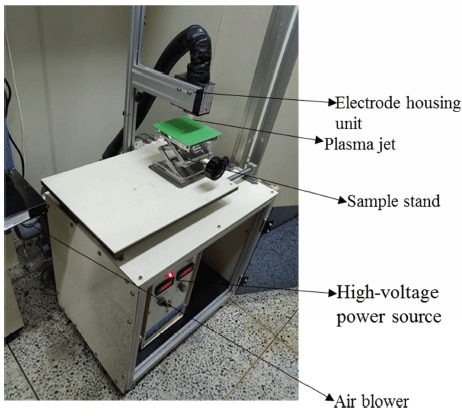
CDPJ generation system, which was designed by the authors, consisted of power supply (output voltage: 20 kV DC; frequency: 58 kHz), electrode assembly, air blower and sample treatment plate, as given in our earlier studies (Kim et al., 2015;Puligundla et al., 2017). The components of the system are illustrated in Fig. 1. A streamer-type corona discharge in air was generated between electrodes (tungsten) under atmospheric pressure conditions. A centrifugal air blower (Ventur Tekniska, Goteborg, Sweden) with a constant speed (3312 rpm) was used for plasma jet creation. The plasma emission slit in electrode housing unit measured 6 × 35 mm. Air velocity at the tip of the electrodes was 2.5 m/s, and a 5 mm interelectrode gap was maintained.
BaP in acetonitrile (10 ppm) test samples were prepared. Drops (10 μL each) of the sample were taken onto glass slides (76 × 26 mm) (a drop on each slide) spread and dried under a laminar air flow for 10 min. Thereafter, the slides were individually placed on the sample treatment plate and treated using CDPJ generated under different input currents (1.00, 1.25, and 1.50 A) and at different sample-to-electrode distances (STED) (15, 25, and 30 mm) for predetermined durations (5, 10, 15, 20, 25, and 30 min). Immediately following plasma treatment, residual BaP from the glass surfaces were recovered using acetonitrile rinse (1 mL for each slide).
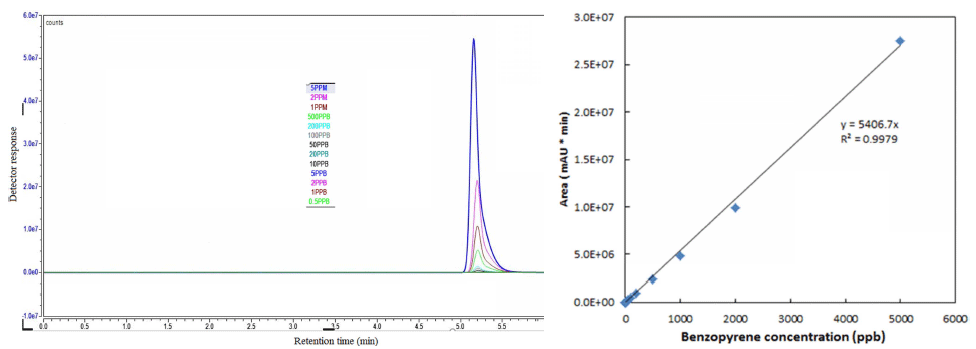
High performance liquid chromatography (HPLC) system (Dionex Ultimate 3000, Thermo Scientific, San Jose, CA, USA) was used for the quantification of BaP before and after the plasma treatment according to the method described previously (Chen et al., 2012). Due to the non-polar nature of BaP, a C18 column (250 × 4.6 mm ID, 5 μm particle size) was used with acetonitrile: water (90:10) as the mobile phase with a flow rate of 1.5 mL/min at the column temperature of 25°C. Residual BaP containing samples were filtered using 0.22-μm PVDF membrane syringe filters (SmartPor, Woongki Science Co., Ltd, Seoul, Korea) before being injected into the column. The amount of sample injected was maintained at 10 μL. BaP detection was performed by using a fluorescence detector at an excitation wavelength of 361 nm and an emission wavelength of 405 nm. BaP concentration was calculated from the peak area using a standard curve. HPLC chromatograms for different concentrations of BaP and calibration curve for BaP are shown in Fig. 2.
BaP concentration was calculated from the detector response to injected samples using a standard calibration curve. BaP degradation was calculated based on the initial concentration of BaP using the following equation:
where Ct = the concentration of BaP at time (t), and C0 = initial concentration of BaP.
For modeling the degradation kinetics of BaP, the appropriateness of the following models was compared.
Log-linear model (Bigelow & Esty, 1920):
Log-linear tail model (Geeraerd et al., 2000):
Weibull model (Mafart et al., 2002):
Weibull-tail model (Albert and Mafart, 2005):
Where,
BaP in food samples, namely sesame and perilla seeds, was induced by their roasting. The roasting was allowed up to a final roasting temperature of 220°C for regular roasted sesame (RRS) seeds and up to 245°C for over roasted sesame (ORS) seeds. In the case of perilla seeds, final roasting temperatures were 210°C and 230°C for regular roasted perilla (RRP) seeds and over roasted perilla (ORP) seeds, respectively.
Roasting-induced BaP in RRS, ORS, RRP and ORP seeds was degraded using CDPJ. Treatment conditions similar to that of BaP degradation on glass slides were used for BaP degradation in RRS, ORS, RRP and ORP seeds. About 10 g of each of the seed samples was exposed to CDPJ at predetermined time intervals (0, 10, 20, and 30 min). Soon after treatment, the samples were extracted with hexane and analyzed to determine the level of BaP degradation.
The concentrations of BaP in oils extracted from the roasted seeds (before and after CDPJ treatment) were also studied. For oil extraction, 10 g sample of each of the RRS, ORS, RRP and ORP seeds was subjected to mechanical pressing using a screw press or expeller (HD-333, Ggaebaksa. Co., Seoul, Korea) and extracted with 100 mL of hexane.
Roasting-induced BaP in the seeds was quantified by the aforementioned method. The following procedure was used in the preparation of samples for the HPLC analysis. Briefly, 10 g of ground seed (flour) /10 g seed oil was mixed with 100 mL of ethyl ether, and the mixture was incubated for 12 h. Then, the ethyl ether of the extract was evaporated, followed by the addition of 100 mL of hexane. The resultant mixture was filtered using Whatman No. 2 filter paper, and the filtrate was transferred to a separatory funnel (I) and 50 mL of N, Ndimethylformamide: water (9: 1, v/v) was added to the funnel and shaken vigorously. The N, N-dimethylformamide-water layer was then transferred to another separatory funnel (II). Then, 25 mL of N, N-dimethylformamide-water was added to the hexane layer of the separatory funnel (I), and the mixture was shaken well and the N, N-dimethylformamide-water layer was added to the separatory funnel (II). The rest of the procedure was the same as that described in our previous study (Lee et al., 2019).
All treatments were performed in triplicate, and the results are represented as mean±standard deviation. Statistical analysis of data was performed using SAS statistical software package (version 9.2, SAS Institute Inc., Cary, NC, USA). Data were analyzed by one-way analysis of variance (ANOVA), followed by Duncan's multiple range test at α= 0.05.
Results and Discussion
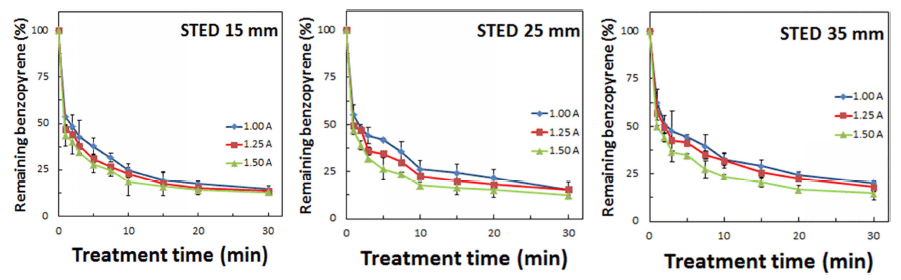
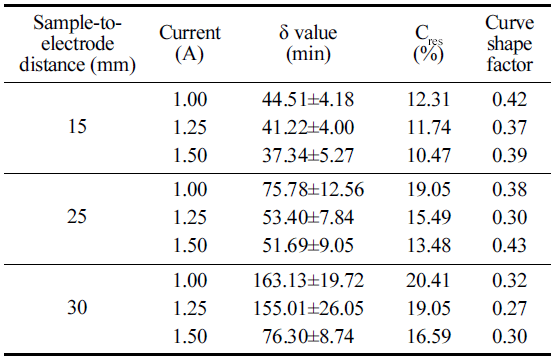
Sample-to-electrode distance influenced the degradation of BaP on glass slides. An increase of the distance linearly decreased the efficiency of BaP degradation (Fig. 3). A steep reduction by up to 50% of initial BaP concentration was observed upon CDPJ (generated using plasma current of 1.5 A) treatment within 1-2 min, at all tested STED (15, 25, and 35mm). Thereafter, the rate of degradation was relatively slowed, ≥85% degradation was noted within 30 min of CDPJ treatment at 15 and 25 mm STED. At the 15 mm distance, residual BaP levels on glass slides following CDPJ treatment (for 30 min) at 1.00 A, 1.25 A, and 1.50 A were 15.10%, 14.86%, and 12.91%, respectively. At the 25 mm distance, residual BaP levels were 15.36%, 14.91%, and 13.11% using CDPJ at 1.00, 1.25, and 1.50 A current, respectively. On the other hand, at a STED of 35 mm, BaP degradation efficiency was relatively low; the levels of residual BaP were 20.24, 18.02 and 14.77% using CDPJ at 1.00, 1.25, and 1.50 A current, respectively. These results indicated that optimal conditions for BaP degradation were CDPJ generation at 1.50 A current and treatment at 15 mm STED.
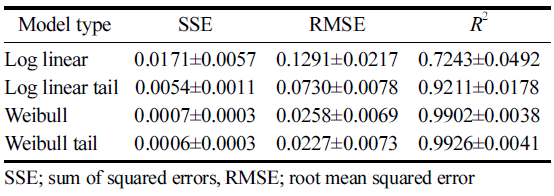
The applicability of selected models, namely log-linear, loglinear tail, Weibull, and Weibull tail, to explain CDPJ-induced BaP degradation was tested. Among these models, Weibull tail model was found to be the best model. The Weibull tail model fitted well to the degradation data. This conclusion was based on based on the values of SSE (sum of squared errors) RMSE (root mean squared error), and R2 (coefficient of determination or fit index). A relatively high R2 value (close to 1) and low SSE and RMSE values for Weibull tail model indicates that the model is the best-fitting model compared with others (Table 1).
|
The same Weibull tail model was used to explain BaP degradation at different process variables. As can be seen from Table 2, relatively low δ value (time to the first decimal reduction) and Cres (residual BaP concentration) values were observed for BaP degradation using CDPJ generated at 1.5 A current and 15 mm STED.
|
Similar results were seen when BaP was degraded on glass slides and in food materials by low-pressure air plasma (LPAP) (Lee et al., 2019). In that study, among selected kinetic models (log-linear, log-linear tail, Weibull, and Weibull tail) to explain LPAP-induced BaP degradation, Weibull tail model was found to be the best model. Based on these results, it can be concluded that nonlinear modelling approaches can suitably explain the degradation pattern of BaP following non-thermal plasma treatment.
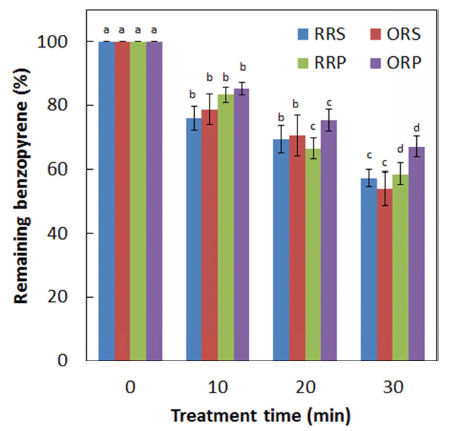
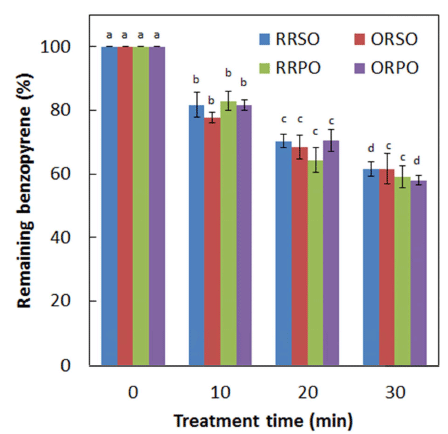
Compared to untreated control samples, a significant (p<0.05) reduction in the levels of BaP in the seed samples was noted upon CDPJ treatment. The average initial levels of BaP in RRS, ORS, RRP, and ORP seeds were 8.51, 51.52, 9.87, and 77.27 μg/kg, respectively. After CDPJ treatment for 30 min, BaP levels in RRS, ORS, RRP, and ORP seeds were reduced by 42.61%, 45.90%, 41.31%, and 32.96%, respectively. Levels of BaP degradation (%) as a function of CDPJ treatment time in RRS, ORS, RRP, and ORP seeds are shown in Fig. 4. In the case of the seed oils, similar BaP degradation patterns were upon the plasma exposure. The average initial levels of BaP in regular roasted sesame oil (RRSO), over roasted sesame oil (ORSO), regular roasted perilla oil (RRPO) and over roasted perilla oil (ORPO) were 3.27, 21.44, 4.43 and 31.24 μg/kg, respectively. Following CDPJ treatment of RRS, ORS, RRP and ORP seeds for 30 min, the levels of BaP in RRSO, ORSO, RRPO and ORPO were decreased by 38.48, 38.37, 40.81 and 41.94%, respectively. Levels of BaP degradation (%) as a function of CDPJ treatment time in RRSO, ORSO, RRPO and ORPO are shown in Fig. 5.
In a previous study, the removal of pyrene and BaP from contaminated water by integrated ozonation-biological degradation techniques was shown (Yerushalmi et al., 2006). They found that, during the simultaneous treatment process (ozonation in the presence of microbial biomass), up to 91% overall removal of BaP following 30 minutes of ozonation (0.5 mg/L ozone); and, during the sequential treatment mode (ozonation as a pretreatment process), increasing to 100% overall removal following 60 min of ozonation (2.5 mg/L ozone). In another study, upon treatment with low-pressure cold plasma (168 W, 1.0 Torr pressure) for 30 min, initial BaP concentration on slides was reduced by 82.7% and by 40-46% in roasted sesame and perilla seeds (Lee et al., 2019). These studies indicate the potential of AOPs for BaP degradation.
The mechanism of BaP degradation seems complex and alter with the type of oxidizing agent. On the application of AOPs, radical reactions and direct reaction pathways have been shown to be predominant in the degradation of BaP in aqueous solution (Miller et al., 2002). Their study showed that radical reactions were prevalent in H2O2-based processes; and about 50% contribution by radical reactions was observed in a H2O2/UV system. On the contrary, direct reactions were prevalent in BaP degradation in a neutral aqueous solution following treatment using O3 and O3/UV systems. It was concluded that acidic conditions favored direct reaction pathways and basic conditions encouraged the radical mechanism of oxidation (Miller et al., 2002). The formation of epoxide products (mono-epoxides and diol-epoxides) upon heterogeneous reaction of BaP with gas-phase O3 has been shown (Zhou et al., 2017).
BaP degradation products, which are formed as intermediates in the oxidative degradation of BaP, could pose a toxic risk. Diol-epoxides, which are formed during BaP oxidation by O3, are known carcinogens (Zhou et al., 2017).
Summary
The plasma reaction conditions for BaP degradation were optimized. Under the optimal degradation conditions, the initial concentrations of BaP on slides were decreased by up to 87.09% in 30 min. The Weibull tail model was found to be best-fit model compared with others to explain the kinetics of BaP degradation by the plasma discharges. In the food samples, following the plasma treatment (at optimal conditions) for 30 min, the average levels of BaP degradation ranged between 32.96-45.90%. As gas plasmas are known to produce different reactive species, radical reactions or oxidation by radical mechanisms might have played a dominant role in BaP degradation.