Introduction
Recently, consumption of natural cheese has increased with change of dietary life and increasing of wine consumption in Korea (Nonghyup Economic Research Institute, 2014). The supply amount of natural cheese has increased from 33,508 ton in 1997 to 103,304 ton in 2016. However, most of the natural cheeses distributed in Korea is imported (Korea Dairy Comittee, 2017). For this reason, milk surplus in Korea has been increased (Korea Dairy Comittee, 2017). Thus, the Ministry of Agriculture, Food and Rural Affairs encourages farmstead milk processing industry to consume milk surplus.
Farmstead dairy product producers believe that raw milk from right after milking can produce high quality and highadded value products (Kim et al., 2014). Since this industry is just initiated, people from the industry may not be familiar with food safety. Hence, contamination with foodborne pathogens in dairy products can occur from dairy environment, handling, and packaging (Pal et al., 2016).
Foodborne pathogens such as Escherichia coli, Listeria monocytogenes, Staphylococcus aureus, Campylobacter spp., Salmonella, spore-forming bacteria, and psychrotrophic bacteria can exist and survive in cheeses (De Buyser et al., 2001; Pal et al., 2016). Bacillus cereus are spore-forming bacteria and exist in dairy farm environments such as soil, feed, and cage (Gopal et al., 2015; Kumari & Sarkar, 2016). The spores have resistance to heat, and thus, they can survive and grow after pasteurization of raw milk (Pal et al., 2016).
Survival and growth of bacteria are affected by the property of cheese such as pH and water activity. Yoon et al. (2016) reported that pathogenic bacteria could grow easily in various cheeses such as Gorgonzola, Emmentaler, and Brie cheeses during ripening because their water activity and ripening temperature are higher than other cheeses (Cogan, 2003; Yoon et al., 2016). Although farmstead milk processing industry was just started to use surplus raw milk, food safety training program and management were not started together. Therefore, the objective of this study was to evaluate the contamination of foodborne pathogens in farmstead cheese.
Materials and Methods
For microbiological analysis, 45 cheeses (Berg, Colby, Cottage, Gouda, Mozzarella, string, Tilsiter, and Quark) were purchased from 16 dairy farms in Korea. Twenty five-gram portions of samples were transferred to sample bags (3M TM , St. Paul, MN, USA), and homogenized with 225 mL of 0.1% buffered peptone water (BPW; Becton, Dickinson, and Company, Spark, MD, USA) for 120 s.
For pre-enrichment, the sample homogenates were incubated at 37 ° C for 24 h. After incubation, seven foodborne pathogens (B. cereus, E. coli, Clostridium perfringens, Salmonella, S. aureus, L. monocytogenes, and Campylobacter spp.) were analyzed.
The pre-enriched homogenates were streaked on Mannitol Egg Yolk Polymyxin agar (MYP agar; Becton, Dickinson, and Company) plus 2.5% egg yolk and 1.8% antimicrobial P. The plates were incubated at 30 ° C for 24-48 h. The presumptive colony was then streaked on MYP agar again and incubated at 30 ° C for 24-48 h.
One milliliter aliquots of pre-enriched homogenates were inoculated into 9 mL of modified EC broth (MB cell, Los Angeles, CA, USA) with 1% novobiocin for enrichment and incubated at 37 ° C for 24 h. The enriched culture was streaked on tellurite-cefixime-MacConkey agar with 1% sorbitol (Becton, Dickinson, and Company) and incubated at 37 ° C for 24 h. The presumptive colony was streaked on Eosin Methylene Blue agar (Becton, Dickinson, and Company) and incubated at 37 ° C for 24 h.
One milliliter aliquots of pre-enriched homogenates were inoculated into 9 mL Cooked Meat Medium (Oxoid Limited, Thermo Fisher Scientific, Carlsbad, CA, USA) for enrichment and incubated anaerobically at 37 ° C for 24 h. The culture was streaked on Perfringens agar (Oxoid Limited) with 0.4% tryptose sulphite cycloserine and 5% egg yolk and incubated anaerobically at 37 ° C for 24 h.
One milliliter aliquots of pre-enriched homogenates were inoculated into 9 mL of Rappaport-Vassiliadis broth (MB cell) for enrichment and incubated at 42 ° C for 24 h. The culture was streaked on Xylose Lysine Deoxycholate agar (Becton, Dickinson, and Company) and incubated at 37 ° C for 24 h.
Ten milliliter aliquots of pre-enriched homogenates were inoculated into 10 mL of 2× Tryptic Soy Broth (Becton, Dickinson, and Company) with 20% NaCl for enrichment and incubated at 37 ° C for 24 h. The culture was streaked on Baird Parker agar (MB cell) with 5% egg yolk tellurite and incubated at 37 ° C for 24 h.
Ten milliliter aliquots of pre-enriched homogenates were inoculated into 10 mL of 2× Listeria Enrichment Broth (Becton, Dickinson, and Company) for enrichment and incubated at 30 ° C for 48 h. One milliliter aliquots of enriched culture were inoculated into 9 mL of Fraser broth (Becton, Dickinson, and Company) and incubated at 37 ° C for 24-48 h. The culture was streaked on PALCAM agar (Oxoid Limited) and incubated at 30 ° C for 48 h.
Ten milliliter aliquots of pre-enriched homogenates were inoculated into 10 mL of 2× Bolton broth with 10% laked horse blood for enrichment and incubated at 37 ° C for 4 h, followed by incubation at 42 ° C for 44 h microaerobically under 14% CO2 and 6% O2. The culture was streaked on Campylobacter blood-free selective medium (Oxoid Limited) and incubated microaerobically at 42 ° C for 48 h.
For identification of bacteria, the presumptive bacterial colonies were selected and analyzed by 16S rRNA sequencing. Single colony on each selective plate was suspended in the Chelex stock solution (Chelex 100 resin, BIORAD, Richmond, California, USA) and heated at 95 ° C for 10 min. Heated suspension were cooled down on the ice for 10 min and centrifuged at 13,000 rpm for 2 min. The supernatant were used for genomic DNA (gDNA). Extracted gDNA was amplified using polymerase chain reaction (PCR) with primers of 518F (5’-CCAGCAGCCGCGGTAATACG-3’) and 800R (5’-TACCAGGGTATCTAATCC-3’). The PCR mixture was formulated with 17 μL of distilled water, 1 μL of gDNA, 1 μL of each 5 pmole primers, 2 μL of 10 mM dNTP, 2.5 μL of 5× SP buffer, 0.5 μL of SP Taq polymerase (Labopass IP-Taq PCR premix, Cosmogenetech, Seoul, Korea). The amplification conditions were 94 ° C for 5 min, followed by 35 cycles of 94 ° C for 30 s, 58 ° C for 30 s, and 72 ° C for 90 s. PCR product was purified by rAPid Alkaline Phosphatase with Exonuclease 1 (Roche, Switzerland). BigDye Terminator v3.1 Cycle sequencing Kit (Applied Biosystems, Foster City, California, USA) was used for sequencing, and then analyzed using ABI Prism 3730XL DNA analyzer (Applied Biosystems).
To enumerate total aerobic bacteria, Pseudomonas spp., coliform, E. coli, and yeast/mold, 1 mL aliquots of homogenates were diluted with 9 mL of 0.1% BPW (Becton, Dickinson, and Company) and the diluents were plated on each selective agar. Petrifilm TM Aerobic Count Plates (3M TM ) was used to enumerate total aerobic bacteria and incubated at 37 ° C for 24 h. To enumerate of Pseudomonas spp., Cetrimide agar (Becton, Dickinson, and Company) was used and incubated at 25 ° C for 24 h. Also, coliform and E. coli were enumerated by Petrifilm TME. coli/Coliform Count Plates (3M TM ) and incubated at 37 ° C for 24 h. After incubation, red and blue colonies with gas bubbles were regarded as coliform and blue colonies with gas bubbles were regarded as E. coli. To enumerate yeast/mold, Petrifilm TM Yeast and Mold Count Plates (3M TM ) was used and incubated at 20 ° C for 5 days.
Results and Discussion
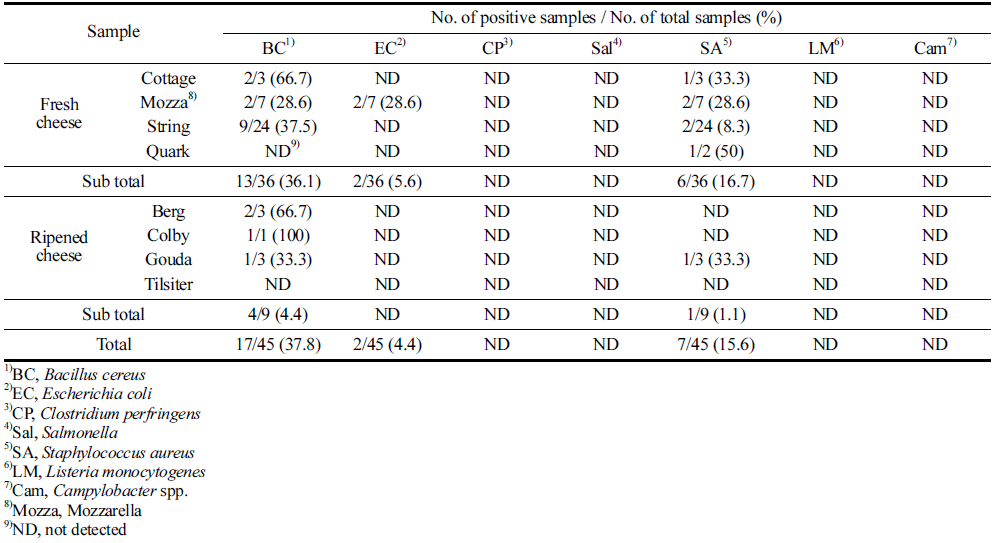
Of 45 farmstead cheeses, B. cereus were positive from 17 samples (37.8%), S. aureus were positive from seven (15.6%) and E. coli were positive from two (4.4%) samples, respectively (Table 1). Among 17 B. cereus isolates, 13 isolates were detected in fresh cheeses (two of Cottage cheeses, two of Mozzarella cheeses, and nine of String cheeses) and four isolates were detected in ripened cheeses (two of Berg cheeses, one of Colby cheese, and one of Gouda cheese). Also, the positive number of B. cereus was the highest in string cheese samples. Seven isolates of S. aureus were detected from six fresh cheeses (one of Cottage cheese, two of Mozzarella chesses, two of String cheeses, and one of Quark cheese) and one ripened cheese (Gouda cheese). Two isolates of E. coli were detected only from fresh cheese (Mozzarella cheeses). Overall, the prevalence of foodborne pathogens was higher in fresh cheeses than in ripened cheeses (Table 1). Other four foodborne pathogens (C. perfringens, Salmonella, L. monocytogenes, and Campylobacter spp.) were not detected in all farmstead cheeses. Also, no bacteria in Tilsiter cheese, one of the ripened cheeses, was contaminated.
Yibar et al. (2017) reported that 11 B. cereus isolates (10.4%) were detected in 106 cheese samples. Also, Messelhäusser et al. (2014) presented that B. cereus was detected in three cheese samples among 131 cheese samples (2.3%). In Argentina, 15 cheese samples of 50 cheese samples (30.0%) were contaminated with B. cereus (Iurlina et al., 2006). Also, Kumari and Sarker (2014) showed that 33% of cheese samples were contaminated with B. cereus in India. These data indicate that B. cereus prevalence in farmstead cheese in Korea is very high, compared to other countries. Hence, there may be high probability for foodborne illness. S. aureus contaminations in foods are usually cross-contaminated by worker’s hands because 20-30% of people have S. aureus on their body (Wertheim et al., 2005; Dancer, 2008). Rosengren et al. (2010) reported that 38 out of 55 raw milk cheeses were contaminated with S. aureus (69.1%) in Sweden. Also, 14 S. aureus isolates (7.7%) were detected in 181 goats’ milk cheeses (Akineden et al., 2008). The prevalence rates of S. aureus in the farmstead cheese in several studies indicate that hygiene of workers, producing farmstead cheese, is very poor, and there is possibility for foodborne illness by S. aureus. E. coli are contamination indicator bacteria and it can be transmitted from feces to food through the environment (Ercumen et al., 2017). E. coli were contaminated in 4.4% of farmstead cheese samples in this study. Similarly, 85 cheese samples (5.7%) of 1,502 cheese samples were contaminated with E. coli in Switzerland (Zweifel et al., 2010). It suggests that farmstead cheese may have fecal contamination, which indicates poor food safety control in farmstead cheese producing environments.
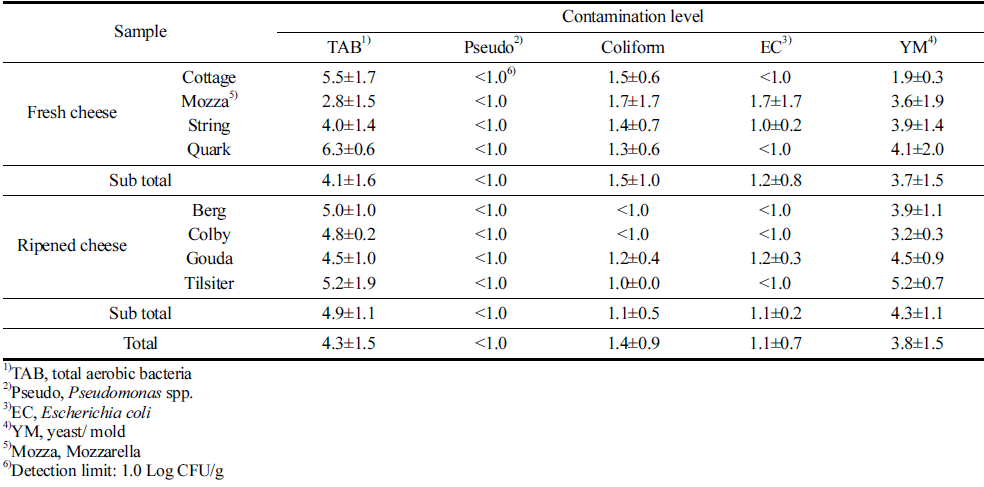
The result of quantitative analysis is shown in Table 2. The mean level of total aerobic bacteria was 4.3 Log CFU/g. The highest level of total aerobic bacteria was observed in Quark cheese at 6.3 Log CFU/g. Also, the mean levels of coliform and E. coli were 1.4 Log CFU/g and 1.1 Log CFU/g, respectively. Coliform were contaminated in Cottage cheese (1.5 Log CFU/g), Mozzarella cheese (1.7 Log CFU/g), String cheese (1.4 Log CFU/g), Quark cheese (1.3 Log CFU/g), Gouda cheese (1.2 Log CFU/g), and Tilsiter cheese (1.0 Log CFU/g). Among eight types of cheeses, Mozzarella cheese, String cheese, and Gouda cheese were contaminated with E. coli at 1.7 Log CFU/g, 1.0 Log CFU/g, and 1.2 Log CFU/g, respectively. Especially, contaminations of coliform and E. coli indicate the fecal contaminations. Thus, the results from this study indicate that the cheeses may have fecal contamination. The mean contamination level of yeast/mold was 3.8 Log CFU/g. Contamination level of Pseudomonas spp., which are spoilage bacteria was below detection limit (1.0 Log CFU/g) in all farmstead cheeses.
|
Villarruel-López et al. (2016) reported that the mean levels of total aerobic bacteria, coliform, and yeast/mold were 7.7 Log CFU/g, 2.9 Log CFU/g, and 4.9 Log CFU/g, respectively. In Brazil, the mean of contamination level for coliform, E. coli, and psychrotrophs were 7.2 Log CFU/g, 5.8 Log CFU/g, and 3.6 Log CFU/g, respectively (Barreto de Deus et al., 2017). Compared with other studies, the mean of contamination level for bacteria in this study was low.
Conclusion
In Korea, the problem of raw milk surplus is continuing despite the increase in consumption of cheese, and thus, the farmstead milk processing industry has been suggested as an alternative to solve the problem. However, the scientific data on the hygiene of farmstead cheese is not very clear. Therefore, the microbial analysis of farmstead cheese was performed. As a result, farmstead cheeses were contaminated with pathogens such as B. cereus, E. coli, and S. aureus. Farmstead cheese in Korea have poor food safety, and there is possibility for causing foodborne illness by consumption of the cheese. Therefore, food safety of farmstead cheese have to be improved dramatically to provide safe farmstead cheese to consumers.