Introduction
Bourbon is the main variety that dominates Rwandan Arabica coffees since its introduction in 1904 (Chemonics International Inc., 2006; USAID, 2010; Promar Consulting, 2011; Nzeyimana et al., 2013). It is one of the natural Arabica coffee varieties and believed to possess nice aroma in addition to the general refreshing effect of coffee. In Rwanda, the areas suitable for Arabica coffee growth are in western region near Lake Kivu, central region and south-eastern region (Harding, 2009; Nzeyimana et al., 2014). The country has recently focused on increasing the production of high quality specialty Arabica coffee whose world demand is also increasing. The nature of coffee (variety) and the area (origin) from which it has been grown are the main factors that affect its unique quality characteristics (Toci & Farah, 2008; Ongo et al., 2012; Buratti et al., 2014).
Coffee quality can be classified into physical, chemical as well as flavor characteristics, but for consumers, flavor is the major coffee quality determinant. Therefore, the pleasant flavor of Arabica coffee is what makes it popular among the two worldwide consumed coffee species (Kathurima et al., 2012; Sunarharum et al., 2014). However, the value of coffee on the market is also affected by physical characteristics such as color, size and defects which influence the acceptability and chemical characteristics of coffee which have a direct impact on flavor as well as on our health. High concentrations of alkyl-methoxy pyrazines for example, have been said to be responsible for cup defects (Czerny & Grosch, 2000), whereas many other volatiles (such as pyrazines, alcohols, aldehydes, ketones, furans, furanones, thiols, pyrans, pyrroles, lactones, pyrimidines and pyridines) and non-volatile components (proteins, lipids, organic acids, melanoidins, alkaloids and soluble fibers) are responsible for the desirable coffee flavor (Farah, 2012).
Coffee chemical constituents such as phenolics (mainly chlorogenic acids), alkaloids (caffeine, trigonelline), diterpenes (cafestol, kahweol), melanoidins and dietary fibers are of great interest for possessing health benefits such as antioxidant activities and also contribute to flavor of the brew (Kitzberger et al., 2014; Vignoli et al., 2014; Gebeyehu & Bikila, 2015; Skowron et al., 2016). Trigonelline is also a known precursor for niacin/nicotinic acid (vitamin B). In addition to chlorogenic acids (CGAs), quinic acid and aliphatic organic acids such as acetic, citric, lactic, and malic acids are responsible for coffee acidity which has an influence on sensory quality (Seo et al., 2003; Franca et al., 2005; Tawfik & El Bader, 2005; Ramalakshmi et al., 2007; Wei et al., 2012; Koskei et al., 2015).
Physicochemical and flavor characteristics of different varieties of Arabica coffees from different producing countries such as chemical properties of Rwanda’s ordinary coffee (Skowron et al., 2016), volatile constituents of Kenyan Arabica coffee (Kathurima et al., 2012), Philippine civet coffee (Ongo et al., 2012), fermented and dried Indonesian Arabica coffee (Baggenstoss et al., 2008; Madihah et al., 2013), cup qualities of Colombia coffee (Maeztu et al., 2001; Rodríguez et al., 2010), Ethiopia, Tanzania, and Guatemala (Akiyama et al., 2007) have been studied. However, no study about quality characteristics of wet processed Bourbon cultivar from Rwanda has been reported. Thus, this study aimed at investigating the physicochemical qualities of this coffee from major producing areas in comparison with Arabica coffees from some established world class origins. The main focus was on some known bioactive compounds (total polyphenol, CGA, caffeine and trigonelline), radical scavenging activity (antioxidant properties), pH and titratable acidity.
Materials and Methods
Eight samples of a wet processed/washed commercial cultivar; Bourbon Mayaguez 139 (BM 139) from seven coffee growing areas of Rwanda selected based on where Arabica coffee growth is suitable were ordered from coffee washing stations (CWS) of four exporters: RWACOF Exports Ltd. (Kigali, Rwanda), SACOF Ltd. (Gakenke, Rwanda), COOPAC Ltd. (Rubavu, Rwanda) and Muhondo coffee company Ltd. (Gakenke, Rwanda). A washed Typica (G2) from Yirgacheffe, Ethiopia and a dry processed/natural Red Bourbon (NY2/ Sc16) from Brazil were bought from GSC International (Seoul, Korea). The growing areas, CWS and producers are indicated in Table 1.
Caffeine (99.9%), 5-caffeoylquinic acid (5-CQA, ≥ 95%), trigonelline (≥ 98.5%), methanol (99.9%), Folin-Ciocalteu’s (FC) reagent, sodium carbonate, gallic acid, 2, 2-diphenyl-1- picrylhydrazyl (DPPH), ethanol, sodium hydroxide (0.1 N) and phenolphthalein indicator were purchased from Sigma Aldrich (St. Louis, MO, USA).
Moisture content of both green and roasted coffee beans was determined in triplicates by measuring loss in mass of whole beans using a forced convection-type oven (WOF-155, Daihan Scientific, Seoul, Korea), according to ISO 6673: 2003 standard method (Reh et al., 2006; Mendonc et al., 2007).
True and bulk densities were determined as previously described (Olukunle & Akinnuli, 2012). Briefly, true density was measured on 10 g of coffee by displacement method using 50 mL of distilled water in a 100 mL graduated cylinder. Bubbles were eliminated by gentle shaking and the change in volume was noted. Bulk density was determined on 10 g of coffee by tapping method (10 times) using 100 mL graduated cylinder. Five measurements were performed for each sample.
Coffee samples were roasted to a light degree in a 500 g capacity drum roaster (Namgaiver, Namyangju, Korea). The roasting process was controlled by a roast profile monitoring software and the same conditions were applied to all samples (sample weight: 200 g, preheating temperature: 180 °C, roasting time: 10 min). Beans were air cooled at room temperature and packed in stand up foil pouches until used. Both green and roasted beans were ground and sieved through mesh No. 30 using a micro fine mill, Culatti Type MFC CZ 13 (BDL, Brno, Czech Republic) for green beans, and R-220 coffee grinder (Fuji Royal, Osaka, Japan) for roasted beans.
The color measurement was performed on both green and roasted coffee grounds using a colorimeter (Chroma meter CR-400, Konica Minolta Sensing Inc., Tokyo, Japan) which was calibrated on a white standard tile. Ten measurements were done for each sample.
Stock solutions of caffeine, caffeoylquinic acid and trigonelline standards were prepared by dissolving 40 mg into 100 mL of distilled water in a 100 mL volumetric flask. The solutions were sonicated to dissolve completely. Concentrations of 2.5-40 ppm were then prepared by 1/2 (v/v) dilution from the stock solutions to obtain calibration curves.
Samples were extracted in triplicates according to DIN 20481 method with some modifications (Naegele, 2013). For both green and roasted coffee, 200 mL of boiling water were added to 1 g of the grounds in a 250 mL Erlenmeyer flask and stirred for 20 min in water bath at 90 °C. The mixture was cooled to room temperature and filtered through Whatman filter paper No. 1 (GE Healthcare UK Ltd., Amersham Place, Buckinghamshire, UK). An aliquot of the extract was filtered through a nylon syringe filter 17 mm, 0.4 μm (National Scientific, Los Angeles, CA, USA) into a 2 mL vial (Agilent Tech., Lexington, MA, USA) and directly injected into the LC-MS system.
Analyses were performed on Agilent 1100 series liquid chromatograph system equipped with an auto sampler, a diode array detector (DAD) and a mass detection system (Agilent LC/MSD VL, Agilent Tech., Lexington, MA, USA). Separation was carried out on reverse phase column: ZORBAX Eclipse Plus, XDB-C18, 4.6 mm × 150, 5 μm (Agilent Tech., Lexington, MA, USA). The experiment was operated on-line by Agilent ChemStation software. Analytical conditions are indicated in Table 2. The contents expressed as percentage by dry weight were calculated from the calibration curves of concentration against peak area.
The concentrations of these two isomers of caffeoylquinic acid were determined using their peak areas and peak area of 5-CQA standard together with their respective molar absorption coefficients according to literature (Trugo & Macrae, 1984; Perrone et al., 2008; Bravo et al., 2012). 5-CQA was identified based on the retention time, UV-Visible and mass spectra of its standard, while the other two isomers were identified according to the order reported in literature and their UVVisible as well as mass spectra.
The total phenolic content was determined using FC reagent diluted to 1/10 and 7.5% sodium carbonate solution as previously described by Rez-Martínez et al. (2010) and Cosoreci et al. (2014). Samples were prepared in triplicates by extracting 1 g of the grounds with 100 mL of 50:50 (v/v) methanol/ water solution for 1 h in a water bath with continuous agitation at room temperature and filtered through Whatman filter paper No. 1. Standard solutions of gallic acid (0.005-0.08 mg/mL) in 50% methanol were prepared by 1/2 (v/v) dilution from a stock solution of 32 mg/100 mL for calibration.
Two and a half milliliters of FC-reagent were added to 0.5 mL of the samples and to 0.5 mL of each of the standard solutions in different test tubes. After 10 min, 2 mL of 7.5% sodium carbonate solution were added and the mixture incubated for 2 h. Polyphenol concentration was then calculated as gallic acid equivalent per gram of sample (GAE/g of dry matter) from the calibration curve of absorbance read at 750 nm against concentration of the standards, using UV-Visible Spectrophotometer (T60 U, PG Instruments Ltd., Lutterworth, UK). Fifty percent aqueous methanol was used as blank.
The samples were prepared as described above for phenolic content determination. 0.1 mM DPPH (2, 2-diphenyl-1-picrylhydrazyl) solution was prepared by dissolving 0.394 mg into 100 mL of methanol. The antioxidant properties were determined by assaying the DPPH radical scavenging activity (RSA) as previously described (Oboh & Omoregie, 2011; Somporn et al., 2011; Pérez-Hernández et al., 2012). 2 mL of 0.1 mM DPPH solution were added to 2 mL of sample extracts and 2 mL of 50% aqueous methanol in different test tubes wrapped in aluminium foil to protect the mixture from light. The tubes were incubated in the dark for 30 min. Changes in the absorbance of the mixtures were measured at 515 nm against 50% methanol as blank using UV-Visible Spectrophotometer. The radical scavenging activity expressed as inhibition percentage was calculated using the equation:
Where, Ao = Absorbance of DPPH alone and
As = Absorbance of DPPH + sample
The acidity of coffee samples was determined according to A.O.A.C 920.20 method (Pez-Galilea et al., 2007). 10 g of coffee grounds were extracted in triplicate by 75 mL of 80% ethanol for 16 h under continuous agitation at ambient temperature in a water bath. Samples were filtered using Whatman filter paper No. 1 and 10 mL of filtrate for roasted coffee and 25 mL for green coffee transferred to Erlenmeyer flask. The filtrate was diluted to 100 mL with distilled water and titrated to an end point of pH 8.2 with 0.1 N NaOH solution using a magnetic stirrer. The acidity was expressed as mL of 0.1 N NaOH/g of coffee (dry matter).
The pH was determined on 25 mL of roasted coffee brews using a calibrated pH meter (pH 210 Microprocessor pH meter, Hanna Instruments, Woonsocket, RI, USA). Five brews per samples were prepared using aero-press coffee makers (Aerobie Inc. Palo Alto, CA, USA) by pouring hot water (93°C) over the grounds in a ratio of 8.25 g of the grounds to 150 mL of water and allowed to stand for 5 min. Three measurements were performed for each brew.
Results and Discussion
Physical characteristics of raw coffee samples are summarized in Table 3. Moisture content of raw coffees was in the range of 7.7-10.2%. Moisture content of raw coffee is an important quality as it influences cost, storability as well as the overall cupping quality of the commodity. Very high moisture content supports the growth of mold whereas very low moisture content may cause inconsistent roasting properties. The ideal moisture content of raw coffee is 8-13% (Mendonc et al., 2007).
Samples from Rwanda showed no significant difference in both true and bulk densities (1.26-1.27 g/cm 3 for true density and 0.669-0.681 g/cm 3 for bulk density) but the difference was significant between Rwandan coffees and the natural Red Bourbon from Brazil which had the lowest density of 1.19 g/ cm 3 . Higher bulk density was also observed for samples from Rwanda (except SKN, SKK and WRB) than washed Typica from Ethiopia. The density of Rwandan coffees obtained in this study is also higher than reported for Ibadan coffees (Olukunle & Akinnuli, 2012). Density measurement reflects the hardness/compactness of the bean which helps to design a proper roast profile to produce the best flavor characteristics of a particular coffee.
The raw Bourbon coffee from Brazil was darker in terms of lightness (L* = 66.5) which was measured on the grounds. This was due to the brown color caused by some pulp and/or parchment attached inside the coffee bean center cut since it was processed by a natural/dry method than the wet processed samples from Rwanda (L* = 68.4-72.1). Among the coffee samples from Rwanda, NGK was the darkest (L* = 68.4). Color especially lightness is one of the criteria used to determine the degree of roast. It is therefore useful to know the color properties of the raw material to begin with in order to optimize the roast. For raw coffee, this visual appearance can also impact its marketability (Ramalakshmi et al., 2007).
Total titratable acidity (TTA) of raw coffee was in the range of 2.1 to 2.3 mL/g of dry matter and raw coffee from Yirgacheffe, Ethiopia presented the highest value (Table 4). The values obtained in this study fall in the range of 2.0-2.9 mL/g reported by Ramalakshmi et al. (2007) for commercial grades of wet and dry processed Arabica and Robusta coffees. Higher values than obtained in this study were found for defective beans. The results obtained however were slightly higher than reported for Harar and Berry coffees by Tawfik & El Bader (2005) perhaps due to varietal, origin or sample preparation (such as ground particle size) differences. Primary acids present in green coffee are chlorogenic acids, quinic acid, citric acid and malic acid (Wei et al., 2012).
The Red Bourbon from Brazil had the highest caffeine content of 18.5 mg/g of coffee on dry weight basis. This was 20% higher than that of NGK (15.3 mg/g) which had the highest caffeine concentration among Rwandan coffee samples evaluated, but NGK showed higher trigonelline concentration of 12.5 mg/g (d.m.) followed by Ethiopian coffee which had 12.1 mg/g (d.m.). The lowest amount of both caffeine and trigonelline was found for ERW (13.4 and 10.3 mg/g, respectively) and WRS (13.8 and 10.7 mg/g, respectively). Caffeine and trigonelline content might be influenced by rainfall and temperature, as these two areas are characterized by low rainfall and higher temperature than other growing areas studied. Caffeine and trigonelline are the main alkaloids found in coffee. Together with phenolics (mainly chlorogenic acids) these alkaloids are bioactive and apart from caffeine, the rest act as precursors for aroma compounds (Farah, 2012; Kitzberger et al., 2014).
In this study, WRB was found to contain the highest total phenolic content (TPC) of 46.8 mg/g (d.m.), whereas SKK had the lowest phenolic content of 38.4 mg/g of coffee (d.m.) than samples from other areas. In terms of caffeoylquinic acid content (chlorogenic acids), NGK showed the highest concentrations of both 3-CQA (12.0 mg/g) and 4-CQA (17.1 mg/g) whereas the sample from Ethiopia had the highest 5-CQA (56.5 mg/g) as well as the total CQA (82.6 mg/g) content than coffees from other areas. Brazilian coffee had the lowest total CQA of 70.1 mg/g.
Chlorogenic acids are the main phenolic compounds found in coffee and caffeoylquinic acids (CQA) are the major subclass reported in literature (Farah, 2012). This study also confirmed the dominance of 5-CQA (> 60% of total CQA) in all coffee samples studied, followed by 4-CQA. The same trend was also found by Skowron et al. (2016) for ordinary coffees from Rwanda but the concentrations were higher than obtained in this study because they were expressed per quantity of dry mass of dry extract.
Phenolic compounds are among the known important antioxidants present in coffee together with melanoidins and Maillard reaction products. However, Isabel et al (Pez-Galilea et al., 2007) did not find a significant correlation between volatile Maillard reaction products and antioxidant properties by DPPH and redox potential methods. The radical scavenging activity of chlorogenic acids and melanoidins was said to be dependent on the type of free radical present. A study by Rez-Martínez et al. (2010) demonstrated that chlorogenic acids (the main phenolic compounds) reacted with Fremy’s salt and not with stabilized radical 2,2,6,6-tetramethylpiperidinooxy (TEMPO), in contrast to melanoidins which indicated a high scavenging activity towards the latter than the former.
The antioxidant activity of melanoidins has been attributed mainly to the low molecular weight compounds linked to melanoidin skeleton. Antioxidant activity of coffee brews has also been shown to be dependent on the preparation method (Pez-Galilea et al., 2007). This is due to the difference in concentration of the bioactive compounds extracted by those methods. The radical scavenging activity of all samples ranged from 89.9% to 91.8% with no significant difference between all other samples except WRS whose RSA was lower than that of three samples (NRL, ERW and SKK).
Caffeine, trigonelline and 5-caffeoylquinic acid were identified by comparing their retention time (Fig. 1), UVVisible absorption spectra and mass spectra (Fig. 2) with those of pure compounds, while concentration calculations were achieved using calibration curves. As mentioned earlier in “Materials and Methods”, the other two isomers of CQA (3- CQA and 4-CQA) were identified based on the order reported in literature (Perrone et al., 2008; Vignoli et al., 2014) and confirmed by their mass and UV-Visible spectra.
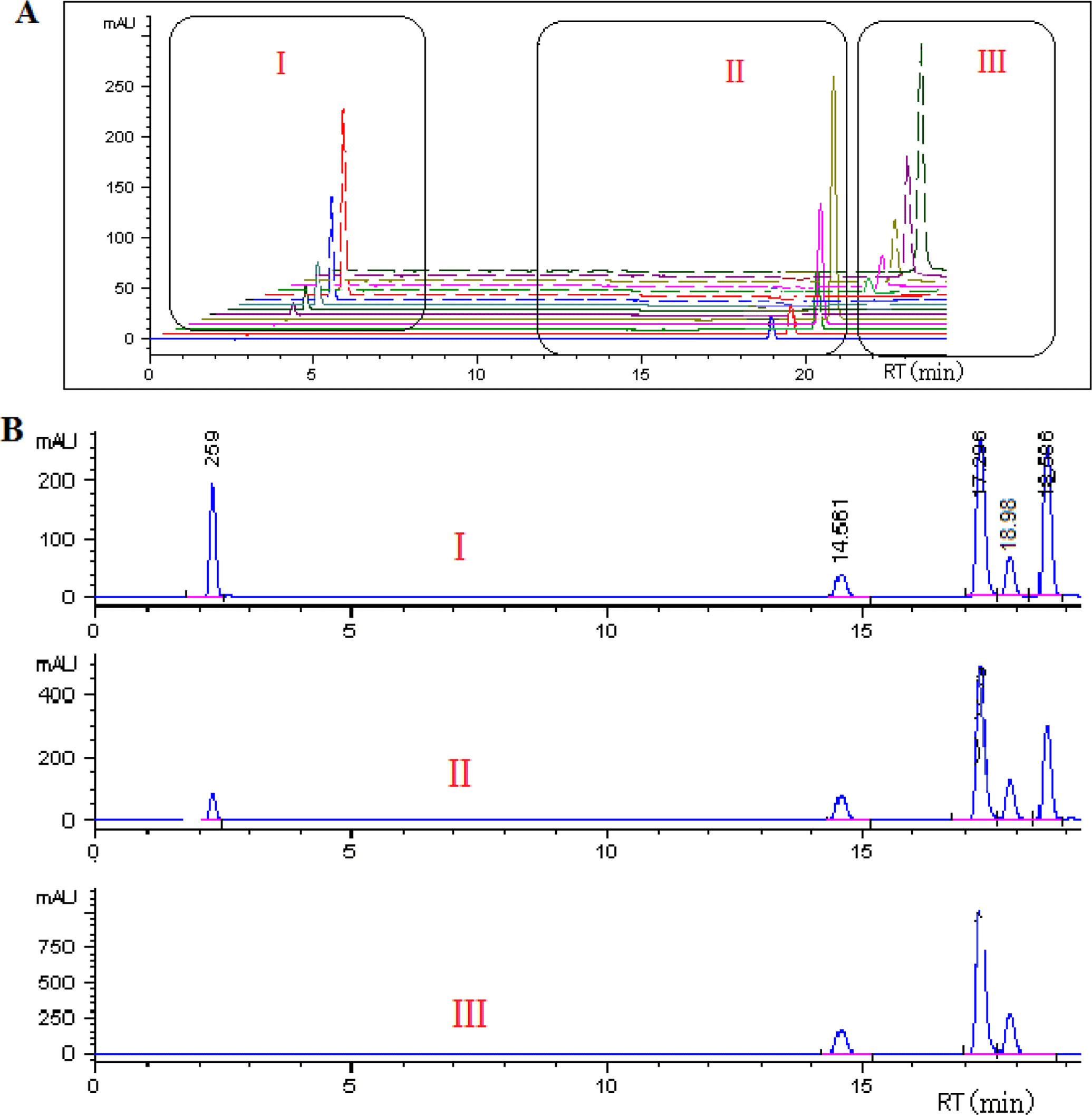
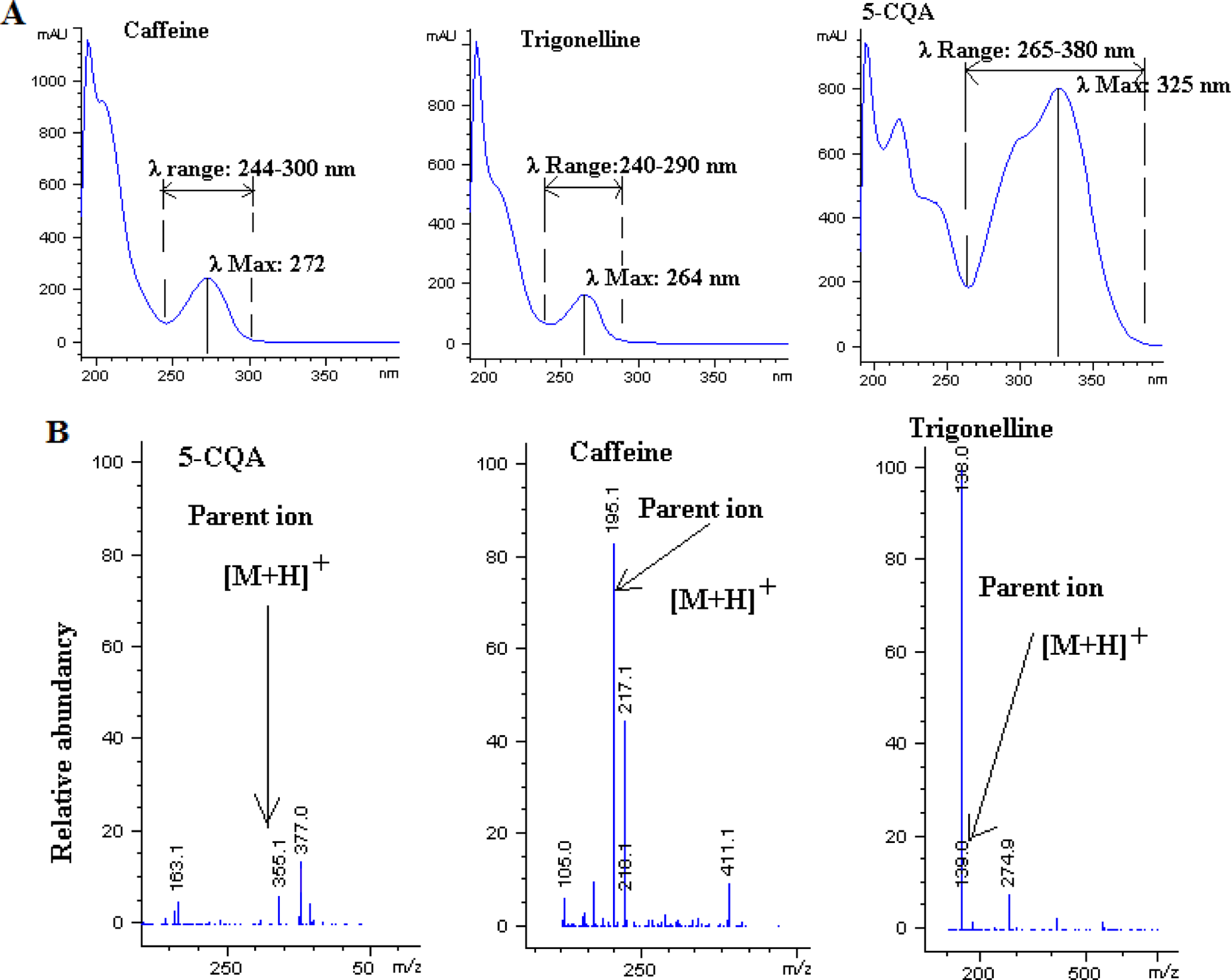
As seen for raw coffee samples, significant differences in physical characteristics of roasted coffees were also observed as indicated in Table 5. After 10 min of roasting, coffee samples lost over 70% of the original moisture content and over 9% of the total weight.
Owing to increase in volume, bulk density decreased to the range of 0.4-0.48 g/cm 3 . Brazilian coffee also showed the lowest density after roasting. It was clearly found that roasted samples with higher moisture content presented higher density. This is because water is denser than roasted coffees. The density values obtained in this study were lower than 0.548- 0.592 g/cm 3 reported by Gebeyehu & Bikila (2015) for Coffea Arabica tip. variety from Sumatra which were roasted by high temperature short time (HTST) and low temperature long time (LTLT) methods for 200-1200 s.
Regarding color changes, the most affected were lightness (L* = 31.1-39.3) and a* (9.6-12.4) compared to b* values (10.0-18.9). Lightness values are in the range used to describe the light degree by Suh et al. (2014). Ethiopian and Brazilian coffees were characterized by more lightness (L*), redness (a*) and yellowness (b*) than Rwandan coffees after roasting. Lightness is commonly used to determine the degree of roast (light, medium and dark) which is directly related to the type of aroma formed. Light roasted coffees are generally used for sensory evaluation to detect the origin character (Akiyama et al., 2008).
TTA of roasted samples was 1.92-2.59 mL/g (d.m.) whereas pH; another measure of acidic properties of coffee brews was found in the range of 4.72-5.02 (Table 6). A good correlation (r = -0.913) was found between TTA and pH values. The pH of coffee depends on the strength of the acids. Rwandan coffees exhibited almost similar acidity properties after roasting but higher than Ethiopian and Brazilian coffees. TTA of Rwandan coffee samples increased after roasting as opposed to Ethiopian and Brazilian coffees whose TTA decreased below that of their corresponding raw beans. This can be attributed to the type, concentration and dissociation properties of the acids present (formed or left after roasting) as a result of the roasting properties of coffees from different origins.
In this study, the pH obtained with the Aeropress extracts of Ethiopian and Brazilian coffees were similar to the range 4.82 to 5.59 reported by Seo et al. (2003) for regular coffees consumed in Seoul coffee shops. TTA values are higher and pH values lower than obtained for Harar and Cherry coffees (Tawfik & El Bader, 2005) and also for soft Brazilian coffees roasted at 200 °C for 1 h (Franca et al., 2005). This is because these samples were roasted longer which led to decrease in the acid fraction as TTA and pH were found to peak at the end of first crack (Wang & Lim, 2012).
These two parameters are functions of extraction temperature and time in addition to coffee cultivar and roasting conditions. For TTA, the end point is another factor that determines the values obtained. The acid fraction of roasted coffees is constituted by various kinds of acids such as chlorogenic acids, quinic acid, short chain aliphatic carboxylic acids (such as citric, acetic, malic, formic and lactic), phosphoric acid and fatty acids. As pointed out earlier, chlorogenic acids are degraded during roasting producing quinic acid and caffeic acid. Other degrading acids during roasting include citric acid and malic acid while acetic acid, formic acid, lactic acid and nicotinic acid are formed (Wei et al., 2012). Some of the coffee acids are volatile contributing to the aroma while others are less or non-volatile contributing to the perceived acidity (taste) of coffee brews.
Caffeine and trigonelline contents after roasting were found to be in the range of 14.8-19.8 and 10.2-11.9 mg/g (d.m.), respectively. Brazilian coffee still had the highest caffeine content after roasting whereas NGK and Ethiopia coffee maintained the first position in trigonelline content. Among Rwandan coffees, NGK together with SKK and WRB showed the highest caffeine content. Coffee from Yirgacheffe, Ethiopian showed similar caffeine content to Wembera coffee of Northwest Ethiopia (Gebeyehu & Bikila, 2015).
In contrast to caffeine which is not affected by roasting process, trigonelline is thermally degraded to nicotinic acid but the conversion rate was said to be low and needs high temperature time conditions (220 °C for 20 min) than used in this study (Taguchi et al., 1985). This might be the reason why a small decrease was noticed. Caffeine appears to increase after roasting due to loss of some other components while remains unchanged. Caffeine and trigonelline contribute to the strength, body and bitterness of the brew. Caffeine and trigonelline contribute not only to the perceived flavor but also possess antioxidant activity. Excellent correlation between caffeine content and DPPH RSA of coffee brews has been reported (Pez-Galilea et al., 2007; Vignoli et al., 2011).
Total phenolic content of roasted coffees ranged from 25.8 to 30.6 mg/g. Brazilian coffee had the smallest amount whereas Ethiopian coffee was neither significantly different from Rwandan coffees nor Brazilian coffee. In this study, roasting coffees up to 10 min (light degree) decreased the total phenolic content by over 30% of the original amount. The antioxidant activity however (89.5-91.5%) seemed to be unaffected by the roasting process as also previously reported (Vignoli et al., 2011). However, after roasting Brazilian and Ethiopian coffees came up with a slightly higher RSA than Rwandan coffees.
Both total phenolic content and RSA are influenced by the extraction methods employed during sample preparation. In this study 50% methanol solution yielded lower TPC than obtained from ground coffee by filter, plunger, mocha, and espresso coffee makers (Rez-Martínez et al., 2010), but in agreement with what was obtained for light and medium roasted Arabica coffees when similar solvent type and concentration were applied in acidic conditions of pH 2.0 (Somporn et al., 2011).
CQA concentration dropped to the range of 32.7-54.3 mg/g (d. m.). Similar results were obtained by Vignoli et al. (2011) with hot water extracts of Arabica coffees (3.53-4.11%). These results are also close to the ones obtained for instant coffee (Trugo & Macrae, 1984). As seen for raw coffees, Ethiopian coffee yielded the highest amount of 5-CQA as well as total CQA. Chlorogenic acid concentration decreased due to thermal degradation by the roasting process and in this study 4-CQA appeared to be the most sensitive whereby over 90% of its initial amount was lost. Similar observation of decrease in chlorogenic acid after roasting was made by Somporn et al. (2011) and Vignoli et al. (2011).
After roasting, browned compounds (melanoidins) have been considered to be the most contributors to the antioxidant properties of coffee brews (Pez-Galilea et al., 2007). DPPH radical scavenging activity was found to be highly correlated with phenolics as well as caffeic acid but no correlation was found with chlorogenic acid (Pérez-Hernández et al., 2012). This means that even though chlorogenic acids are said to be the main phenolics present in coffee, they may not be the antioxidant phenolic fraction. Vignoli et al. (2011) also did not find any correlation between 5-CQA and antioxidant activity but rather found strong correlation between DPPH RSA and phenolics.
Conclusions
This study indicated that the wet processed Bourbon from Rwandan was less caffeinated than the dry/natural processed Bourbon from Brazil and characterized by low CQA content than the wet processed Typica from Ethiopia. However, the roasted wet processed Bourbon from Rwanda had higher acidity properties than Brazilian and Ethiopian coffees studied. Since both Bourbon and Typica belong to Coffea Arabica, this study indicated that coffee quality may vary even within the same variety from different or same country due to differences in environmental conditions, processing as well as agricultural practices applied in different coffee growing areas.