Introduction
Three-dimensional (3D) printing has great potential for tissue engineering because it provides a rapid and robust approach to assemble functional tissue in vitro (Richards et al., 2013). Scaffolds have been manufactured according to material and output using various methods, including fusion deposition modeling (FDM) (Hutmacher et al., 2001), selective laser sintering (SLS) (Ko et al., 2007), laminated object manufacturing (LOM) (Feygin & Pak, 1999), and stereo lithography apparatus (SLA) (Hull, 1986). Among them, FDM has been widely used in the past decade due to its high durability, precision, and relatively low cost (Gibson et al., 2015; Nyberg et al., 2017a). FDM allows sufficient resolution needed for structure and pore design, and utilizes many bioactive materials applied to FDM to create a footplate with similar stiffness to the original bone (Nyberg et al., 2017b).
Currently, the standard treatment for bone defect is using autologous graft. However, due to limited supply of adequate bone and bone defects caused by trauma, scaffolds are created for tissue engineering (Karp et al., 2003). In the emerging tissue engineering field, porous scaffolds are used to support bone tissue cells to replace and complement current approaches. Scaffolds or three-dimensional constructs are structures that support cell proliferation and tissue to maintain their functions (Hutmacher, 2000). These scaffolds can be made from a wide variety of natural and synthetic materials. Poly (lactide-co-glycolide), hydroxyapatite (HA), polymers, ceramics, and metals have been applied to make artificial bones (Kim et al., 2006; Venkatesan et al., 2015). Among them, poly(ε-caprolactone) (PCL) is a hydrophobic biodegradable polymer approved by the USFDA and has been applied in biotechnology. Natural polymers such as chitosan, collagen, cellulose, chitin, fibrin, and alginate are biocompatible and nontoxic substances with good physiological activity to human body (Gillette et al., 2010; Sinha et al., 2015). However, they have limitation because their catabolic rate is difficult to control and their mechanical properties are weak (Kuijpers et al., 1999). Synthetic polymers are also suitable for use as medical materials because they have no cytotoxicity. In addition, they can regulate the catabolic rate (Kim et al., 2015). Although these synthetic polymers can provide structural integrity, it is difficult to process them. In addition, they have little ability to promote bone formation (Greenwald et al., 2001). Due to these problems, the design and synthesis of these substance need to be reviewed before they are applied to human body (Okamoto & John, 2013). In this regard, natural materials not only complements existing materials, but also presents advantage as environmentally sustainable bio-filaments.
Among various natural materials, rice bran, an agricultural by-product, has been reported to be able to enhance cell proliferation and protein synthesis and promote bone regeneration (Lim et al., 2014a). It is a polymer-based agricultural byproduct that contains gamma-aminobutyric acid (GABA) which inhibits cholesterol in the blood and promotes the secretion of growth hormone. In addition, rice bran contains vitamins, potassium, minerals, iron, magnesium, protein, and natural antioxidants such as tocotrienols and oryzanol (Sharif et al., 2014). Rice bran serves as a source of numerous individual bioactive compounds. Recently, it has been reported that rice bran has many pharmacological effects (Friedman, 2013), including anti-obesity (Jin-Nyoung et al., 2012), anticancer (Somintara et al., 2016), and antioxidant activities (Samad & Haleem, 2017). Rice bran can also enhance differentiation through activating BMP-2 and p38 MAPK in C2C12 cells (Moon et al., 2014). However, to the best of our knowledge, no previous study has reported rice bran products as biomaterials for bone formation by increasing cellular immunity or eliciting biological responses.
Therefore, the objective of the present study was to fabricate rice bran biocomposite as a potential bone-graft analogue using FDM-based 3D printing. In this work, a new 3D printing system toward composite filament was developed using agricultural by-product rice bran via precision extrusion process technology. It was called “r-PCL” in this study. Our hypothesis was that complex bio-filament containing agricultural by-product rice bran could enhance PCL formation, a negative aspect of PCL alone. Our results showed that composite bio-filaments appeared to be a promising way to produce solid bio-filaments using FDM-based 3D printing technology. This study will foster innovation throughout 3D printing system toward composite filament called “bio-filament”.
Materials and Methods
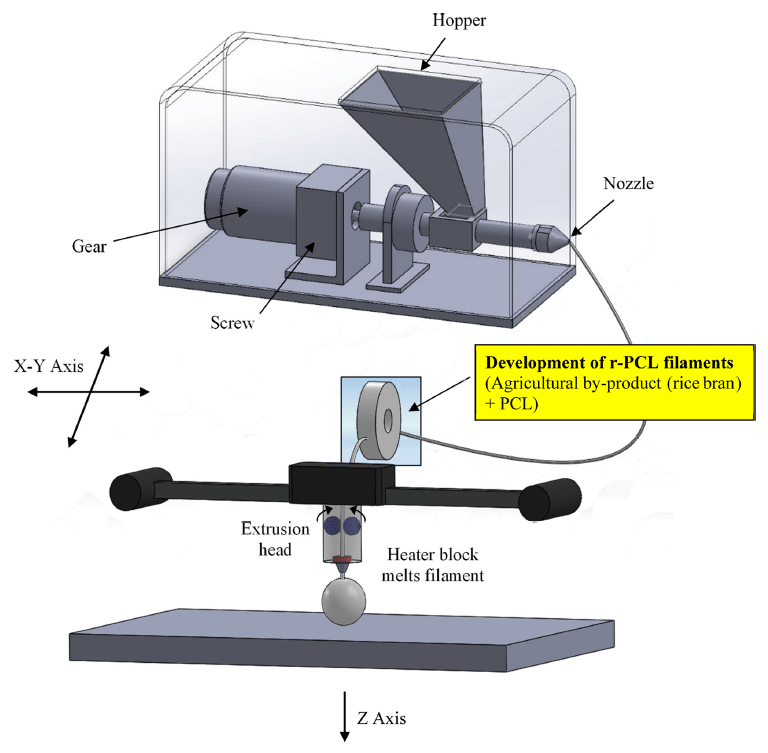
In this study, four kinds of samples were prepared in the following procedure. First, rice bran (Korea Philgreen, Seoul, Korea) (5, 10, or 25 g) was added to 300 g of chloroform (Duksan, Seoul, Korea) and placed at room temperature for 24 h. After dissolving, the chloroform containing rice bran was mixed with 50 g of PCL (Sigma-Aldrich, USA) and placed at room temperature for 24 h. The sample was extracted with a 1.75 mm nozzle extruder (Philibot, Leejo, Changwon, Korea) at 24 rpm and 150 °C (Table 1). PCL was used as a control. To ensure nontoxicity of biological samples, efficient extraction was performed at high temperature. Schematic depiction of FDM technology used in 3D printing of bio-filament composites for this study is shown in Fig. 1. Specifications of the extruder are listed in Table 2. Products of this process were defined as r-PCL 10%, r-PCL 20%, and r-PCL 50%.
| Operation System | Temperature & DC controller |
|---|---|
| Temp. | 23-230°C |
| Speed | 0-24 rpm |
| Ext Rat | 2 cm/1 s |
| INP | 1.65-3.0 mm |
| Nozzle | 1.75 mm |
| Power | 220 V, 200 W |
| Hopper | 400 G |
| Dimension | 19 cm (H) * 27 cm (W) * 44 cm (L) |
| Input (g) | Speed (rpm) | Temp (°C) | Diameter (mm) | |
|---|---|---|---|---|
| PCL | 70±21) | 24 | 150±2 | 1.66±0.2 |
| r-PCL 10% | 70±2 | 24 | 150±2 | 1.20±0.1 |
| r-PCL 20% | 70±2 | 24 | 150±2 | 1.48±0.2 |
| r-PCL 50% | 70±2 | 24 | 150±2 | 1.33±0.2 |
Microstructural and particle size analyses of these biofilament composites were performed using a field emission scanning electron microscope (FE-SEM, S-4300, HITACHI, Tokyo, Japan). All samples were coated with platinum using an ion sputter coater (E-1010, HITACHI, Tokyo, Japan) for 240 s at 0-30 mA. The thickness of the composite filament was measured with the FE-SEM. Chemical analysis was performed using energy-dispersive x-ray spectroscopy (EDX) spectroscopy. Average and standard deviation were reported for each value.
Stress-strain analysis was performed to analyze mechanical properties of these bio-filament composites and conventional filament. Stress-strain analysis was performed using QUASAR 5 (GALDABINI, Cardano al Campo, Varese, Italy). Compressive strength of bio-filament composites were measured with a texture analyzer (TA-XT Plus, Stable Micro Systems Ltd, Surrey, UK). These tests were repeated three times. Breaking point displacement was recorded with load cell of 10 kg capacity. Cross-head speed of 1 cm/min was used for tensile strength test.
NIH3T3 cells (KCLB 21427, Korean Cell Line Bank, Seoul National University College of Medicine, Seoul, Korea) were cultured in Dulbecco’s modified eagle’s medium (DMEM, Hyclone, Atlanta, GA, USA) in a culture dish at 37°C with 5% CO2. The medium was supplemented with 1% antibiotics and 10% fetal bovine serum (FBS, Hyclone). Cytotoxicity was analyzed with WST-1 assay (EZ-Cytox Cell Viability Assay Kit, Daeillab Service Co., Ltd, Seoul, Korea). The amount of toxic material extracted from the filament when a 1 cm 2 filament was inserted into the body was assumed to be 100%. PCL, r-PCL 10%, r-PCL 20%, and r-PCL 50% filaments were added to 2 mL cell culture medium and cultured at 37 °C with 5% CO2 and 95% relative humidity. For the control group, pure medium was used. The extract concentration of PCL in the experimental group was 0, 25, 50, 75, or 100%. Optical density (OD) was measured at wavelength of 450 nm using an ELISA reader (VersaMax, Molecular Devices, Sunnyvale, CA, USA). Average and standard deviation were reported for each value.
The water contact angle of sample was measured with a video contact angle system (Phoenix 600, Scientific Gear, Fairfax, VA, USA). Water was used as testing liquid. Droplet size was set at 10 μL. Water contact angle was measured as a tangent line to the interface of the water droplet in the sample. The same test was repeated five times for each sample. Values were averaged for water contact angle.
All statistical analysis were carried out using SAS for Windows v8.2 (SAS Institute Inc., Cary, NC, USA). Statistical significance between control and treatment groups was compared with two-way analysis of variance (ANOVA), Duncan’s multiple range test, and Mann-Whitney Rank Sum Tests. Data are presented as mean±SD. Statistical significance was considered at *p<0.05.
Results and Discussion
PCL is an aliphatic polyester. It is one important biodegradable polymer that gains increasing interest due to its inherent biocompatibility and technological properties (Aithani & Mohanty, 2006a). It is often used in medical devices because it is a well-known biodegradable polymer that can be hydrolyzed by enzymes in the body (Holland et al., 1987). Especially, it has excellent compatibility with other polymers. Many studies have been carried out to improve its properties through polymerization or synthesis (Liao et al., 2012; Son et al., 2015). These composites have been actively studied to induce bone formation by improving their biocompatibility, biodegradability, and strength (Pan et al., 2012; Weisgerber et al., 2016). The main motivation of the present study was to design novel bio-filament composites with rice bran and find value-added application of agriculture by-products.
Representative FE-SEM morphological images of biofilament composites containing both PCL and rice bran were shown in Fig. 2. Results ascertained that the material extracted from both rice bran and PCL was well mixed after removing the organic solvent. Their average diameter was 1.3 ± 0.1 mm. Filaments of r-PCL showed very smooth and uniform surface regardless of the concentration of rice bran used.
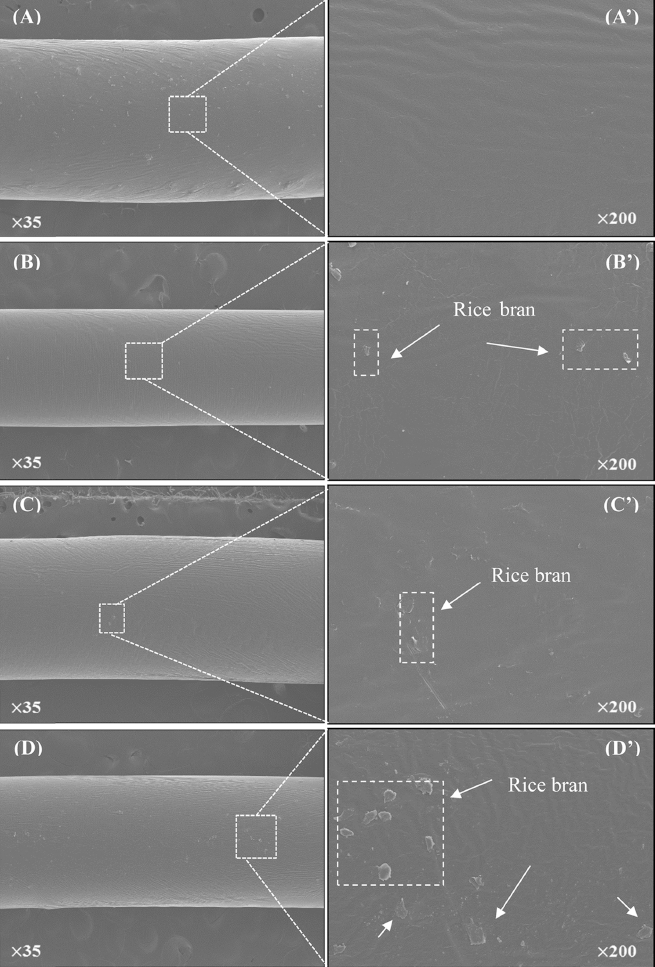
To have successful fabrication of r-PCL, it is necessary to control extract rate and operational parameters such as pellet input amount, temperature, and processing speed. Without proper control of these parameters, high-quality filament cannot be obtained (Koh et al., 2006). To analyze the effect of processing conditions, Aithani et al. have kept the processing speed constant at 120 rpm with temperature of 150 °C after mixing corn gluten powder and PCL. To analyze the effect ofprocessing conditions, treated materials (corn gluten powder and PCL) at speed constant at 120 rpm with temperature of 150 °C were found to be more consistent than those at lowtemperature (Aithani & Mohanty, 2006b). Based on these results, treatment temperature of 150 °C was also used in this experiment. Extracting filaments with a constant diameter would be very important for the experiment. Because irregular surfaces are more easily affected by bacteria and foreign matters than smooth structured materials (Kim et al., 2011), bio-filaments might be more resistant to infection than conventional PCL.
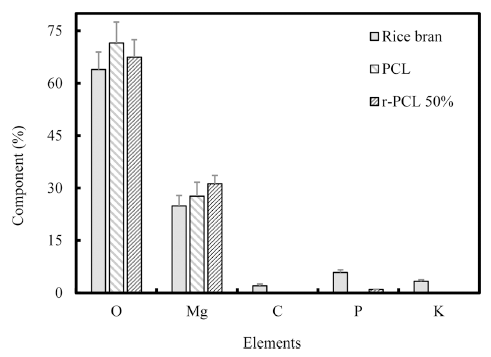
Chemical element distribution in these extracted filaments was analyzed by EDX (Fig. 2). The experiment was repeated three times. The r-PCL 50% filament contained more carbon (C), potassium (K), and phosphorus (P) than theose of the conventional PCL filament. Detailed results were shown in Table 3. Compositions of P in PCL filament and r-PCL filament were 0.72±0.03% and 0.98±0.03%, respectively. Compositions of K in conventional PCL and r-PCL were 0.02± 0.005% and 0.18±0.01%, respectively. These results indicated that r-PCL filaments contained more essential elements of human body such as C, K, P than those of PCL filaments. Thus, the possibility of r-PCL as a biomaterial was confirmed.
Stress-strain curve and tensile strength of r-PCL are shown in Fig. 3 and Fig. 4, respectively. The stress-strain diagram shows important information about mechanical properties of filaments (Kim et al., 2009a). For PCL filament, yielding point occurred when the strain was about 18%. After that, the load was not increased any more (Fig. 3). Yielding points of r-PCL 20% filaments and r-PCL 50% filaments occurred when strains were about 8% and 10%, respectively. For r-PCL 10% filament, the strain was about 11%. After that, the load was not increased any more. This result showed that r-PCL 20% filament had higher elasticity property, compared to r-PCL 10% or r-PCL 50% filament. The stress-strain behavior observed in this study could be further customized due to the flexibility of direct deposition fabrication technique. Tensile strengths of r-PCL 10%, r-PCL 20, and r-PCL 50% filaments were 7±0.84, 15±2.4, and 13±1.8 MPa, respectively (Fig. 4). Tensile strength of the conventional PCL filament was 11±1.34 MPa. The tensile strength of r-PCL 20% filament was about 1.4 times higher than that of the conventional PCL. However, tensile strength of r-PCL was not proportional to the percentage of rice bran added.The tensile strength of these bio-filament composites was first increased with increasing rice bran concentration from 10% to 20%. It was then decreased with further addition of rice bran up to 50%. These results demonstrate that optimal content of r-PCL is needed to achieve the best tensile strength.
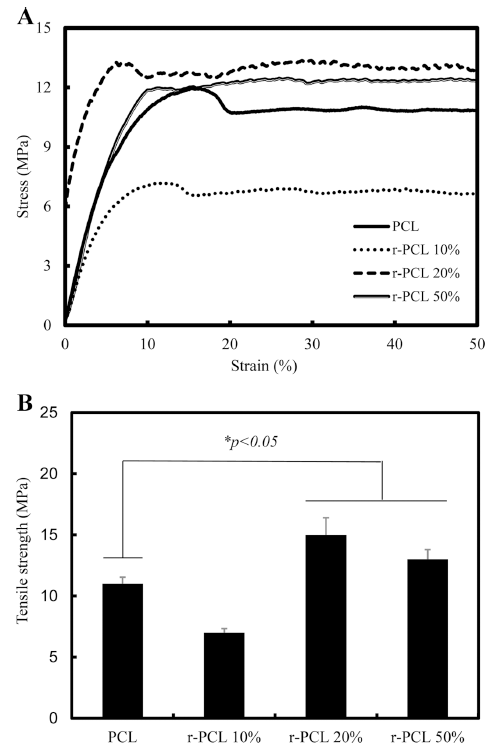
Our results were similar to results of previous reports about PCL scaffolds (Causa et al., 2006). The synthesis of HA/PCL hardens the material and increases the modulus of elasticity. Similarly, it has been reported that the addition of eggshells slurry can cause more intermolecular interaction between particles and macromolecules to ensue network formation (Dadhich et al., 2016a).
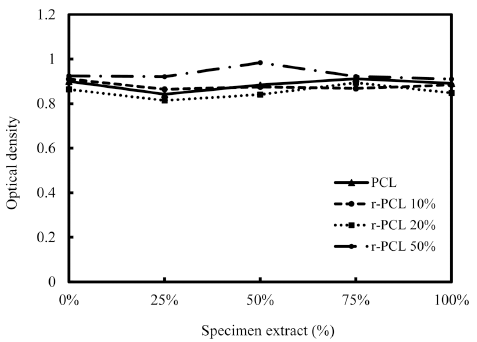
Results of MTT cytotoxicity test for r-PCL composites were shown in Fig. 5 according to the content of rice bran added. The average value of OD in 0% extract as a control was 0.9248 while the average value of OD in 100% extract was 0.9011. The two were not significantly (*p>0.05) different in OD value. These results indicated that r-PCL filament had no cytotoxicity. Biodegradation or biocompatibility of these biofilament composites was not measured in this study. However, previous in vitro studies using MG63 cells have shown that bio-filaments do not cause toxicity or inflammation. In fact, they have positively increased cellular metabolic activity and bone formation (Lim et al., 2014b).
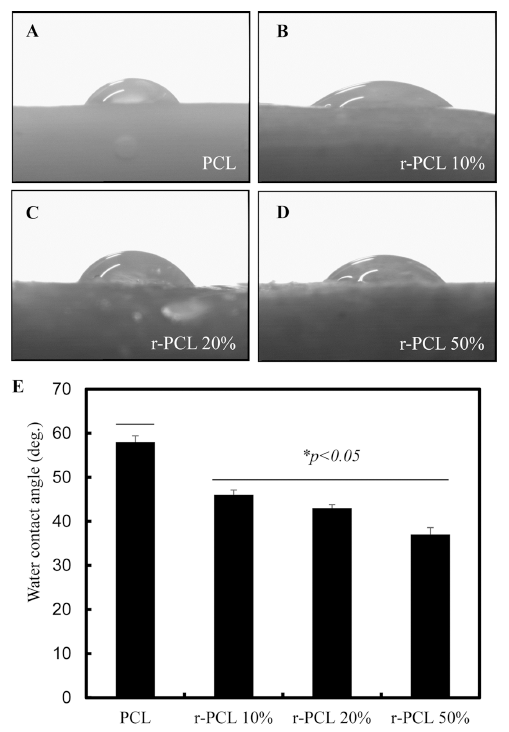
Water contact angle gives hydrophilicity to material. Such surface property can greatly influence cell adhesion and growth on such material (Kim et al., 2009b). Results of water contact angles of bio-filament composites were shown in Fig. 6. Specifically, the contact angle of PCL filament was 58°. These contact angles of r-PCL 10%, r-PCL 20, and r-PCL 50% filaments were decreased (*p<0.05) to 46°, 43°, and 37°, respectively. Bio-filament composites at higher contents of rice bran demonstrated more hydrophilic property. Surface porosity is one factor that can promote cell attachment and proliferation. It has been previously reported that the porous structure of artificial support can improve the biological performance over traditional scaffold structures (Dadhich et al., 2016b). We have previously studied the porous structure of rice bran (Lim et al., 2014c) and demonstrated the potential of rice bran bio-filament composites as a precursor for bone implant. These findings provide a new paradigm for the use of agricultural by-product in bone grafting. Results of this study provide new way to fabricate of environmentally friendly composite bio-filaments. Our results also suggest that 3D platform blending together with agricultural biomaterials can enhanced mechanical and biological properties of biofilaments and significantly reduce the surface hydrophobicity of PCL.
Conclusions
Synthesis of scaffold provides the best way to fabricate environmentally friendly composite bio-filaments. In this study, rice bran bio-filament was analyzed by FE-SEM and EDX. To examine biological responses of cells to such bio-filament, NIH3T3 cells were used. Mechanical properties of these biofilaments were also compared to those of conventional PCL. The average diameter of these bio-filament composites was 1.3±0.1 mm. EDX results indicated the presence of organic compounds in these bio-filaments. Tensile strength of r-PCL 20% filament (15±2.4 MPa) was about 1.4 times higher than that of conventional PCL (11±1.34 MPa). Water contact angle of r-PCL filaments was lower compared to that of control PCL. In addition, water contact angle of r-PCL 10% (46°) was higher than that of r-PCL 50% (37°). Results of this study demonstrated that bio-filament composites exhibited positive response in cytotoxicity with enhanced mechanical and biological properties compared to that of control. Plus, rice brain significantly decreased the surface hydrophobicity of PCL. In conclusion, bio-filament composites are easy to prepare with suitable characteristics as potential biomaterials for tissue engineering. They might benefit agriculture, medical, and food engineering fields. Bio-scaffolds made with such manufacturing technique could facilitate tissue reconstruction, repair damaged tissues and organs, and produce artificial organs or living tissue structures.