Introduction
Salt is an essential element for human survival, because of its important functions, such as the regulation of water content in the body and electrical signaling in the nervous system (Caldwell et al., 2000). The analysis of trace heavy metals in salt has been important to various foods and related research fields. Thus, because of the accelerating environmental pollution near the sea, it is necessary to monitor heavy metals in sea salt produced by the natural evaporation of sea water. Heavy metals are considered to be one of the main sources of environmental pollution due to their significant harmful effects to ecology. In proportion to human activity, such elements are easily diffused to the environment, and they are persistently accumulated in various objects including foods and the food chain. To easily monitor the diffusion of trace heavy metals into the environment, a few techniques have been developed, such as square wave anodic stripping voltammetry (SWASV) (Kefala et al., 2003; Kadara & Tothill, 2004; Maroulis et al., 2007; Xu et al., 2008), X-ray fluorescence (Radu & Diamond, 2009), chemodosimetry (Wu et al., 2007; Mahapatra et al., 2011), and immunoassay kit.
In sea salt, the detection of Cd and Pb is performed by ICP. Aside from other inorganic materials, the heavy metal analysis of sea salt has been performed below a concentration of 3 wt%, a lower concentration than sea water (Leonhard et al., 2002), because the high concentration of Na+ directly influences the plasma temperature and the ionization potential shift of ICP (inductively coupled plasma) equipment, resulting in the difficulty of measurements (Falk et al., 1997).
As an alternative method, SWASV is appropriate for measuring heavy metals under high Na+ concentrations, such as saturated NaCl solutions. Among the electrochemical approaches, this method is mainly employed for its easy manufacturing, low cost, and high sensitivity. SWASV measures the electrochemical signals through the three steps. First, the analyte is deposited onto a working electrode from a stirred solution. This is the pre-concentration step which yields the lowest detection limit of all voltammetric methods. Second, the steering is stopped to keep a stabilized state. This is the reset period. Third, the deposited analyte is redissolved (stripped) from a working electrode. This is the stripping step. The major advantage of SWASV method is the high sensitivity due to the pre-concentration step. The detection limit can reach nanomolar concentration. The technique is useful for the analysis of much diluted solutions down to 10−10 M (Skoog et al., 1998).
Bismuth-coated electrodes, as an environmentally-friendly electrode, have been used for stripping analysis (Rodilla et al., 1998; Sun et al., 1999; Bard & Faulkner, 2001; Wang, 2005). Along with the advancement of SWASV techniques, measurement of heavy metals by employing various types of working electrodes have been introduced, such as a glassy carbon electrode (Wang et al., 2000; Wang et al., 2001; Mcgaw & Swain, 2006; Xu et al., 2008), a carbon screen printing electrode (Choi & Kim, 2009), a magnetic electrode coated with superparamagnetic iron oxide nanoparticles (Yantasee et al., 2008), and an on-chip planar bismuth electrode (Zou et al., 2008). In addition, the development of pretreatment processes could diversify the measureable objects and be applied to various research fields, such as toys, soil, and foods, and so on.
The salts ingested for human survival could be the source of heavy metal exposure. Despite sea salt being an important food, the analysis of residual heavy metals in sea salt by an electrochemical method has not been reported yet. Heavy metal detection is still limited to sea water containing 3.3% NaCl (Svancara et al., 2006; Guell et al., 2008). Here, we report the detection of heavy metals in high concentrated NaCl solutions by MRDES in conjunction with SWASV.
Materials & Method
All of the solvents used in this experiment (ACS grade) were purchased from Sigma-Aldrich (St. Louis, MO, USA and used without further purification. Acetic acid (CH3COOH), sodium acetate (CH3COONa), and sodium chloride (NaCl) of trace metal grade (99.99 %) were purchased from Sigma- Aldrich. The water used was high purity Milli-Q water (Millipore, > 18 MΩcm). A pure copper rod (diameter 2 mm) and alloy rods (diameter 2 mm and 3 mm) comprising copper and tungsten were obtained from Yu Myoung Metal Co. (Seoul, Korea). The ICP standard solutions of cadmium, lead, and bismuth (1000 μgmL−1) were purchased from AccuStandard Inc. (New Haven, CT, USA). A glassy carbon rod (diameter 3 mm) was purchased from Nilaco Co. (Tokyo, Japan). Carbon powder and mineral oil were obtained from Sigma- Aldrich. Polyetheretherketon (PEEK, diameter 6 mm) and industrial salts were purchased from a domestic market. Edible salts formed by the natural evaporation of seawater were obtained from Tae-Pyeong Salt Farm Co. (Shinan, Korea).
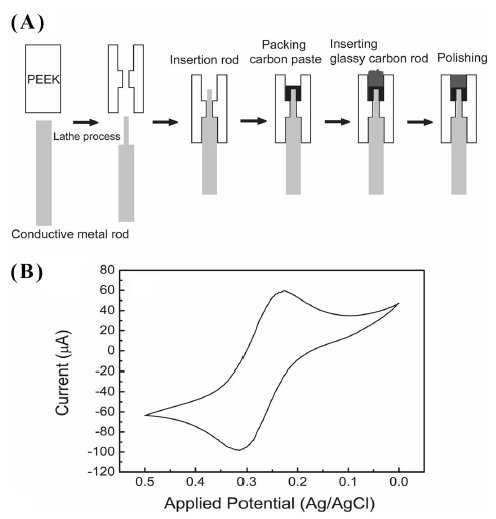
Fig. 1A shows the fabrication process of a manufactured glassy carbon electrode (MGCE) as a working electrode. Polyetheretherketon (PEEK) was used for the main body of a working electrode. The end of the alloy rod (diameter 3 mm) was treated by lathe work until it reached 2 mm diameter. The conductive metal rods (diameter 2 mm) were used without further mechanical treatments. Both facets in the PEEK rod were made with holes adequate for the diameter of the two types of copper rods and the glassy carbon rod, followed by tightly inserting the conductive metal rod from the lower hole, and then densely packing the carbon paste mixed with mineral oil from the upper hole. After coarsely polishing both sides using sand paper, the glassy carbon rod cut to a length of 5 mm was inserted into the upper hole. The protruding part of the glassy carbon rod was polished with sand paper until it was flat. Afterward, an elaborate polishing was successively performed by 1 μm and 400 nm Al2O3 slurries. MGCEs were manufactured by two types of copper rods for the electrical signal transmission: a pure copper rod (diameter 2 mm, flexible) and copper alloy rods (diameter 2 and 3 mm, hard). The electro-deposition step supplying a key role in the ASV measurement was performed during the rotation of a working electrode. As a vertical rotor, MGCE gives rise to the eccentric motion (shown in Fig. 2B) with respect to the rotational axis due to its configuration which shows the difference in diameter between the PEEK body (diameter 6 mm) and the metal rod (diameter 2 and 3 mm). When the metal rod (diameter 2 mm) was applied, an excessive eccentric rotation was produced, and the stripping analysis could not be performed due to the mechanical collision between the electrochemical cell and the working electrode. On the other hand, applying a copper alloy rod (diameter 3 mm) could largely decrease the extent of the eccentric rotation. The results of the stripping analysis are shown in Fig. 3-6.
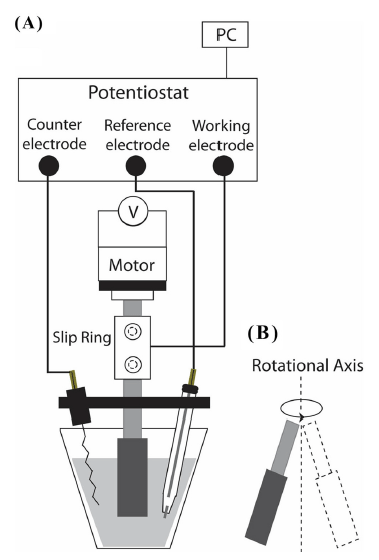
As shown in Fig. 2, a slip ring was introduced to transmit electrical signals between the working electrode and the potentiostat. Passing through the slip ring, the end of metal rod was connected to the stepping motor fixed at 1000 rpm. In the electrochemical cell, a three-electrode system, comprising an MGCE as the working electrode, a platinum coil wire as the counter electrode, and Ag/AgCl (saturated KCl solution) as the reference electrode, was used.
The SWASV was performed with the MRDES in connection with a commercial potentiostat (Epsilon, Bioanalytical System Inc., West Lafayette, IN, USA). Prior to the electrochemical analysis, the working electrode was manually polished with 400 nm Al2O3 and, rinsed with ethanol and water. To enhance the redox reaction of working electrode, the electrode was immersed in 0.1M H2SO4 solution for approximately 5 min. The counter electrode was cleaned with a torch lamp flame until the color changed from orange to blue. In the electrochemical cell (20 mL), three electrodes were immersed in the solutions containing lead, cadmium, and 500 μgL−1 bismuth. The solutions were used without degassing. Bismuth, cadmium, and lead were simultaneously deposited on the working electrode upon when the applied voltage versus Ag/AgCl reached -1.2 V, while rotating at 1,000 rpm. The deposition time was 120 s. Following the equilibrium period of 10 s, the voltammogram was recorded in the potential range of -1.2 V- 0 V by applying a square waveform with an amplitude of 25 mV, a frequency of 15 Hz, and a potential step of 4 mV. Prior to measuring the next samples, the bismuth film and the deposited heavy metals on the working electrode were removed under the conditions of 0.3 V for 30 s.
The excess sea salts were dissolved in Milli-Q water, and then treated by sonication. Three types of saturated solutions were used for the stripping analysis: a saturated solution prepared by trace-metal-grade NaCl (SSTM), saturated solutions prepared by edible salt (SSE), and saturated solutions prepared by industrial salt (SSI). The pH values of SSTM, SSE, and SSI were 5.5, 8.3, and 8.3, respectively. The pH values were controlled by the NaOH and HCl solution. Before measurements, the saturated solutions were treated by a 400 nm pore syringe filter to remove any residual impurities. Limit of detection (LOD) was calculated based on the standard deviation of peak current (SD) and the slope of calibration curve (S) according to the formula: LOD = 3.3 (SD/S) (Stone & Ellis, 2017).
Results and Discussion
MGCE, as a detector of redox species, is a major part of the stripping analysis system. It is necessary to examine its proper performance in response to redox reactions. To ascertain the electrochemical responsivity, a cyclic voltammogram was recorded in a 6 mM K3Fe(CN)6 and 1.0M KNO3 solution. The potential was scanned from 0 V to 0.5 V at a scan rate of 100 mV. Fig. 1B shows the electrochemical response of cyclic voltammetry. At the characteristic potential corresponding to the redox reaction of , anodic and cathodic peak potentials were observed at 0.31 V and 0.22 V, respectively, and the separation between the two potentials was 0.09 V. In addition, the observed anodic and cathodic currents were 74 μA and 82 μA, respectively. These results indicated that MGCE could be applied to a working electrode in electrochemical analysis.
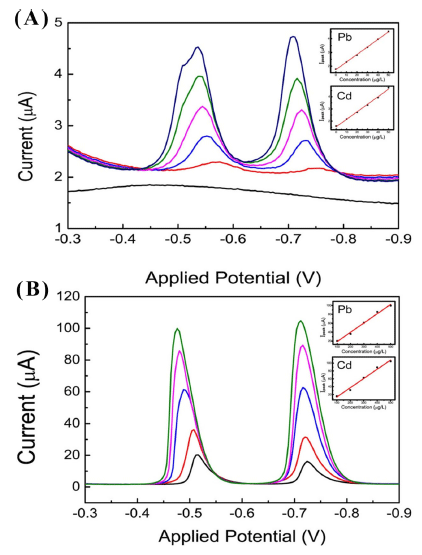
Fig. 3A shows the typical signals of SWASV by increasing Pb and Cd concentrations by 10 μgL−1. The peak intensity increased with the increase in Pb and Cd concentrations. The peak potential (Ep) of Pb and Cd was observed at -0.54 V and -0.72 V, respectively. The calibration plots of Pb and Cd inserted in Fig. 3A were quite linear with correlation coefficients of 0.993 and 0.996, respectively. The slopes of the linear plots were 1.10 ± 0.04 Ag−1 for Pb and 0.95 ± 0.02 Ag−1 for Cd, which indicates that the responsivity of MGCE is more sensitive to Pb than Cd. The stripping signals by increasing the Pb and Cd concentrations in a 100 μgL−1 step are shown in Fig. 3B. The Ep of Pb and Cd was observed at -0.48 and -0.72 V, respectively. The calibration plots of Pb and Cd shown in the insertions of Fig. 3B were quite linear with correlation coefficients of 0.9877 and 0.9820, respectively. The detection limits was 1.8 ± 0.02 μgL−1 for Pb and 4.4 ± 0.06 μgL−1 for Cd. The observed Ep was almost the same value compared with that of the previously reported results (Wang et al., 2000). The shift in Ep could be observed as increasing the concentration. This shift arises from the adsorption of the complexional metal ions on the active surface of the working electrode, which is consistent with a previous report (Banks et al., 2005). The observed peaks were sharp, symmetric, and distinctly separate between the two peaks, offering a convenient quantitative analysis of the two metals. Over a wide concentration range, the linearity and responsivity of MRDES could be applicable for the in-situ monitoring of aqueous media.
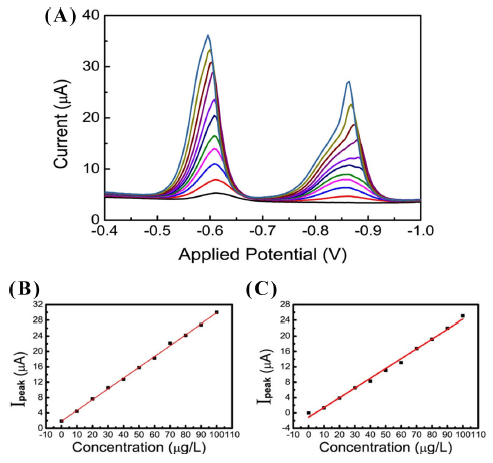
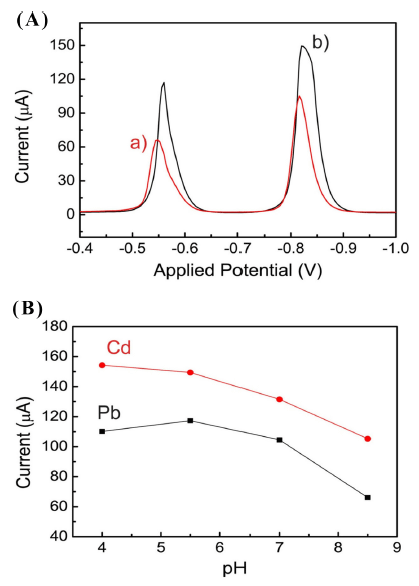
Fig. 4 shows the voltammograms in SSTM. In a concentration range of 0-100 μgL−1, the stripping signals were measured by increasing the Pb and Cd concentrations in a 10 μgL−1 step. As the Pb and Cd concentrations increased, the peak intensity of the current was similar to the results of the acetate buffer. The Ep of Pb and Cd was observed at -0.6 V and -0.86 V, respectively. The calibration plots were quite linear with correlation coefficients of 0.9987 for Pb and 0.9938 for Cd. The detection limit was 3.6 μgL−1 ± 0.18 for Pb and 3.9 μgL−1 ± 0.37 for Cd. To further investigate the effect of pH, a stripping analysis was performed under various pH levels at constant concentrations of Cd and Pb. In acidic condition at pH 4.5 acetate buffer pH range, the feature of stripping signal is more clear and sharp and the intensity of signal increase, compared with basic conditions. The results are shown in Fig. 5. As pH increased, the peak currents of Pb and Cd decreased. Minor shift in Ep was observed. The intensity of the current was largely dependent on pH; on the other hand, Epf was not subjected to the influence of pH. The small current drop was observed in the acidic condition (pH 4-5.5) compared with the basic condition. As the pH increased from 5.5 to 8.5, a large current diminishment was observed. At pH 8.3, the ratio of current drop compared with that of pH 5.5 was 0.60 for Pb and 0.73 for Cd, respectively.
As a reference test, ICP-mass spectroscopy was employed to determine the Pb and Cd concentrations of industrial salts and edible salts. In the edible salts, the measured concentrations of Pb and Cd were 180 μgL−1 and 20 μgL−1, and in industrial salts, they were 590 μgL−1 and 20 μgL−1, respectively (n: number of experiments, n = 3).
Fig. 6 shows the anodic stripping voltammograms of SSE and SSI. The measurements of Pb and Cd were performed without adding any supporting electrolyte. The pH value of SSI and SSE was 8.3. The observed Ep of Pb was -0.6 V, close to the measured value in Fig. 4A. Stripping signals of Pb could be observed in the SSIs, however, stripping signals of Cd could not be observed in other saturated solutions. The main reasons that Cd signals could not be observed are the concentration decay and the current drop as described in Fig. 5. The concentration decay signified that NaCl concentration in a saturated solution is one-fourth of an original NaCl solid.
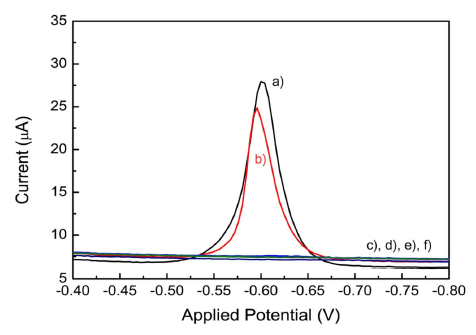
Based on the linear regression equation derived from Fig. 4 and considered the ratio of the current drop, the peak current values was converted into their concentrations.(1)(2)
YPb and YCd are the peak current values of Pb and Cd, respectively. The Pb concentrations obtained in SSI were 622 ± 7.7 μgL−1 (Figure 6a) and 553 ± 6.9 μgL−1 (Figure 6b), which are closely in accord with the concentration of ICP. The results were in the good agreement between the two experimental methods, and they indicate that the MRDES functioned properly for the stripping analysis in the acetate buffer and high-concentration NaCl solution.
Conclusion
In the high-concentration NaCl solution, the residual content of heavy metals in sea salt was analyzed using SWASV method. As increasing in pH from 4.0 to 8.5, the peak current significantly decreased. The detection limit was 3.6 ± 0.18 μgL−1 for Pb and 3.9 ± 0.37 μgL−1 for Cd in saturated NaCl solution. The MRDES, demonstrating linearity and responsivity, was favorably applied for the determination of heavy metals in sea salt. Additionally, the system is an alternative tool for recovering the demerit of ICP occurring in high Na+ concentrations, and it can be applied to various food safety fields requiring the in-situ monitoring of heavy metals.