Introduction
Bombyx mori, a major species for sericulture, has been cultivated since ancient times. Recently, the applications for silkworm byproducts have not been limited to making fabrics using silk from cocoons. In addition to eating silkworm pupae, several important functional materials have been discovered in silkworm byproducts. Control of blood glucose concentrations has been shown to be induced by silk protein hydrolysate (Kim et al., 2007; Jung et al., 2010; Do et al., 2012), improvements in male sexual function have been shown to be induced by male silkworm pupal extracts (Oh et al., 2012), enhancements in cognitive function have been shown to be induced by degraded silk fibroin proteins (Kim, 2005; Kang et al., 2013) and protection against alcoholic hepatotoxicity has been reported by silkworm excrement powder (Kim, 2008). Moreover, silkworm is a good source of protein, fibroin, amino acids, peptides, 1-deoxynojirimycin, and pigments have been found to protect hyperlipidemia and hyperglycemia (Kim, 2008). Silk protein fibroin has been widely explored for many biomedical applications including cell support matrixes for hepatocytes, fibroblasts and scaffolds (Gotoh et al., 2004). Recently, we have developed a technique for processing mature silkworm larvae that were formerly inedible because of the hardness of their silk glands (Ji, 2015). Mature silkworm larvae have an enlarged silk gland and other organelles that are enriched with the previously identified functional materials mentioned above (Kim, 2005; Kim et al., 2007; Kim, 2008; Jung et al., 2010; Do et al., 2012; Oh et al., 2012; Kang et al., 2013).
The liver plays an important role in many bodily functions from protein production and blood clotting to cholesterol, glucose (sugar), and iron metabolism. Hepatocellular carcinoma (HCC) is known to be occurred by chronic hepatitis. Chronic inflammation affects continuous cell division and causes random genetic injury, leading to hepatocarcinogenesis (Okuda, 1992; Tsukuma et al., 1993). Diethylnitrosamine (DEN) is a representative hepatocarcinogen found in various foods, alcohol, and tobacco smoke. The main source of N-nitrosamines is protein, which is produced from nitrate precursor. The gastric acid can activate the amines to N-nitrosamines (Janani et al., 2010; Jayakumar et al., 2012). Various hepatocarcinogen such as DEN cause liver damage and promote hepatocellular carcinogenesis in animal models. DEN can increase cell proliferation or hepatocellular necrosis in the liver, thereby inducing the initiation of carcinogenesis (Maeda et al., 2005; Glauert et al., 2010; Qiu et al., 2011). DEN also has been reported that induce the production of reactive oxygen species causing cellular damage and oxidative stress (Amin et al., 2011; Ghosh et al., 2012; Jayakumar et al., 2012). In this study, we investigated the protective effects of three types of SMSP pretreatment against acute liver damage induced by DEN treatment. Pretreatment with SMSP significantly abrogated the upregulated serum biochemical markers of hepatotoxicity, histopathological change, and inhibited pro-inflammatory enzyme.
Materials & Methods
Silkworm larvae were reared by feeding mulberry leaves in the spring season of 2016 at the National Academy Agricultural Science. The silkworm varieties used for the experiment were Bombyx mori White-jade cocoon strain (also known as Baekokjam), Golden-silk cocoon strain and Light-green cocoon strain (also known as Yeonnokjam) (Lee, 1984). SMSP was made as previously published (Ji, 2015). Briefly, live mature larvae of the Bombyx mori were immediately smothered for 130 min at 100 °C with steam using an electric pressure-free cooking machine (KumSeong Ltd., Boocheon, Korea) followed by freeze-drying with a freeze-drier (FDT-8612, Operon Ltd., Kimpo, Korea) for 24 h. Larvae were then grinded using a hammer mill (HM001, Korean Pulverizing Machinery Co. Ltd., Incheon, Korea) and a disk mill (Disk Mill01, Korean Pulverizing Machinery Co. Ltd.). The lengths of particles of SMSP were shorter than 0.1 mm. The SMSP was stored at -50 °C and then used for formulating diet for mouse.
AIN-76A and three SMSP containing AIN-76A diets were purchased from DBL (Umsung, Korea). Briefly, White-jade, Golden-silk or Light-green SMSP were mixed with powdered AIN-76A, then this mixture were dried and prepared as a pellet chow by DBL (Umsung, Korea). Three different amounts of SMSP were added to AIN-76A; low dose (1 g/kg of AIN-76A for the treatment with 0.1 g/kg of mouse body weight), middle dose (10 g/kg of AIN-76A for the treatment with 1 g/kg of mouse body weight) and high dose (100 g/kg of AIN-76A for the treatment with 10 g/kg of mouse body weight). Three different concentrations of mouse diet were similarly formulated.
The C57BL/6 mice were purchased from Orient bio (Seoul, Korea). Mice were fed sterilized commercial pellet diets (AIN- 76A) or diets containing SMSP and sterile water ad libitum and housed in an air-conditioned room on a 12-h light/dark cycle at a temperature of 24 °C. Animals were handled in an accredited animal facility in accordance with international policies of animal center of the CHA University. Mice were randomly divided into eleven groups (N=6) as follows; (a) control group: animals were fed with commercial diet, AIN- 76A, (b) DEN group: mice were fed with AIN-76A for two weeks and DEN (100 mg/kg, i.p.) was applied 18 h before the end of this experiment, (c) DEN + 0.1 g/kg of White-jade group, (d) DEN + 1 g/kg of White-jade group, (e) DEN + 10 g/kg of White-jade group, (f) DEN + 0.1 g/kg of Golden-silk group, (g) DEN + 1 g/kg of Golden-silk group, (h) DEN + 10 g/kg of Golden-silk group, (i) DEN + 0.1 g/kg of Light-green group, (j) DEN + 1 g/kg of Light-green group, (k) DEN + 10 g/kg of Light-green group. Mice were fed each SMSP containing AIN- 76A diet for two weeks and DEN (100mg/kg, i.p.) was applied 18 h before the end of this experiment DEN and other chemicals were supplied from Sigma-Aldrich (St. Louis, MO, USA).
All mice were sacrificed by taking blood via cardiac puncture under carbon monoxide anesthesia after treatment with DEN for 18 h. The livers were rapidly removed and rinsed with PBS and kept in ice. The materials were stored at -80 °C until they were analyzed.
For histopathological assessment, fixed liver portions were embedded in paraffin blocks, followed by cutting 4 μm sections and mounting them on glass slides for hematoxylin-eosin (H&E) staining. Hepatocyte ballooning is a form of liver cell injury recognized as a swollen hepatocyte with a rarefied cytoplasm.
Hepatic injury was evaluated biochemically by measuring the activities of alkaline phosphatase (ALP), alanine aminotransferase (ALT/GPT) and aspartate aminotransferase (AST/GOT) in serum using Hitachi automatic analyzer 7600-210 (Hitachi High-Technologies Corporation, Tokyo, Japan).
This assay was performed as previously described (Park et al., 2014). Briefly, the liver was homogenized with ice-cold cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma Aldrich, St. Louis, MO, USA). After 20 min of incubation, samples were centrifuged at 10,000 × g for 10 min. Supernatants were then collected. Proteins in lysates were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes, which were incubated with cyclooxygenase- 2 (COX-2, Thermo Fisher Scientific Inc., Waltham, MA, USA), inducible nitric oxide synthase (iNOS, Santa Cruz Biotechnology Inc., Dallas, TX, USA) and β-actin (Santa Cruz Biotechnology Inc.), washed, incubated with peroxidaseconjugated secondary antibodies, rewashed, and then visualized using an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK).
Results are expressed as the mean±standard deviation. The data were analyzed by one-way analysis of variance with SPSS 10.0 (SPSS Inc., Chicago, IL, USA) and the statistical significance between groups was determined by Duncan’s multiple range test. Statistical significance was accepted at p<0.05.
Results & Discussion
To examine the hepatoprotective role of SMSP, we used a commonly used DEN-induced acute liver injury mouse model. DEN is usually employed to induce experimental hepatotoxicity and HCC. The high dose of DEN, acting as an initiator in HCC, induces cell death and compensatory proliferation in the liver (Maeda et al., 2005; Glauert et al., 2010; Qiu et al., 2011). DEN exposure can activate adjacent Kuppfer cells to induce compensatory proliferation, which is critical process in HCC induced by DEN (Maeda et al., 2005; Glauert et al., 2010; Qiu et al., 2011). In this study, 100 mg/kg of DEN was injected and this dose was enough to induce cell death and initiate carcinogenesis in the liver (Bingul et al., 2013). Single dose of 100 mg/kg DEN was intraperitoneally injected into 8-week old C57BL/6 mice after pretreatment with diet containing three types of SMSP for two weeks. Mice were sacrificed 18 h following injection and liver damage was assessed. At the end of the experiment, the body weights and the liver weights were measured and summarized as relative weight in Table 1. In the DEN group, the liver had a slightly brighter color than that of the vehicle control. The percentage of liver weight versus body weight in DEN-treated mice was significantly decreased (vehicle 5.63±0.50% vs. DEN group 5.02±0.28%, P < 0.05). It has been suggested that DEN causes liver atrophy, possibly through enhancing hepatocyte death. As shown in Table 1, however, DEN-mediated decrease in liver weight was inhibited by SMSP preadministration. Pretreatment of high dose of each SMSP significantly prevented the liver weight decrease induced by DEN treatment, suggesting that 10 g/kg of White-jade, Golden-silk and Light-green SMSP could protect the liver injury induced by DEN. Among these, White-jade SMSP showed the most effective prevention of liver weight loss induced by DEN treatment.
To investigate the hepatoprotective effect of each silkworm powder, we performed the histopathologic observation of livers. Normal liver structure was shown in no treatment groups histopathologically (Fig. 1a). H&E stained liver sections demonstrated that DEN exposure for 18 h provoked necrosis in the central and portal areas, infiltration of neutrophils and lymphocytes and distinct edema around the central vein, and portal areas in DEN group (Fig. 1b). Considerable swelling cytoplasm and vacuolar degeneration in parenchymal cells were also observed. However, pretreatment with diet containing all three SMSP inhibited the hepatocyte damage induced by DEN in a dose-dependent manner. Pretreatment of high dose of each SMSP exhibited almost intact portal areas and decreased parenchymal necrosis (Fig. 1e, h and k).

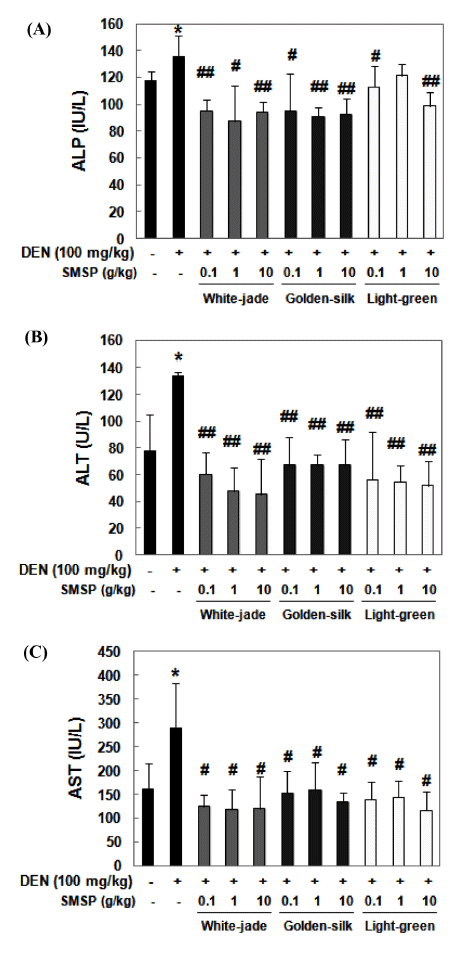
To determine the DEN-induced liver damage, the levels of three indicators in the serum, ALP, ALT and AST were measured by the colorimetric method. ALT/GPT is almost exclusively found in the liver. When the liver tissue is diseased or damaged, additional AST and ALT are released into the bloodstream, which increases their activities. Thus, measuring serum levels of AST or ALT is a valuable tool in the diagnosis of liver damage (Mathews et al., 2014). ALP is an enzyme in the cells lining the biliary ducts of the liver. ALP levels in plasma rise with large bile duct obstruction, intrahepatic cholestasis, or infiltrative diseases of the liver. Similar to ALT and AST, ALP is associated with the plasma membrane of hepatocytes adjacent to the biliary canaliculus. Obstruction or inflammation of the biliary tract results in an increased concentration of the ALP in the circulation (Bleibel et al., 2007). The activities of ALP, ALT and AST were significantly induced in DEN-treated mice, which reflect liver damage, whereas significantly suppressed in diet containing each SMSP-fed mice than DEN-treated mice (Fig. 2A-C). Preadministration of White-jade SMSP showed the most effective suppressive effect on ALP, ALT and AST activities. Altogether, these results demonstrate that SMSP pretreatment might have protective effects on DEN-induced hepatotoxicity.
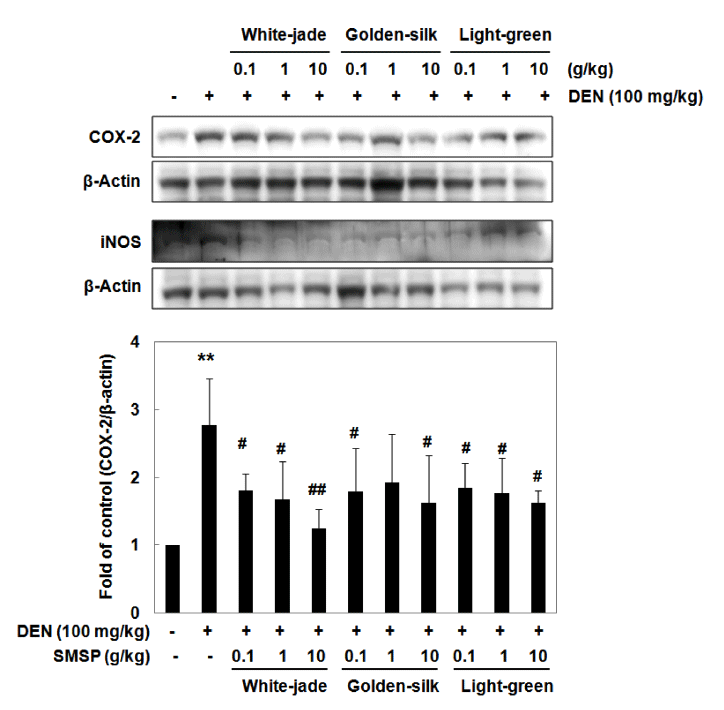
Some animal studies have been reported to explain the link between inflammation and hepatocarcinogenesis according to liver injury (Luedde et al., 2007; Haybaeck et al., 2009; Schneider et al., 2012). Following liver injury and the release of inflammatory mediators primarily from Kupffer cells, neutrophils and monocytes are recruited to the liver by these mediators and subsequently amplify the inflammation response by secreting more inflammatory mediators (Liaskou et al., 2012). To study the roles of SMSP on inflammatory response in liver tissue, the expression of COX-2 and iNOS, a representative inflammatory enzyme was determined by Western blot analysis. The expressions of COX-2 and iNOS were highly induced by DEN treatment. The expressions of both proteins, however decreased in the diet containing each SMSP-fed groups in a dose-dependent manner (Fig. 3). Compared to the control mice, administration of DEN led to elevation of COX-2 and iNOS levels, however, administration of SMSP, even at a dose of 0.1 g/kg, prior DEN resulted in attenuating these inflammatory enzymes in mice (Fig. 3). White-jade SMSP pretreatment showed the most effective inhibitory effect on the expressions of COX-2 and iNOS. These data indicate that pretreatment with SMSP can effectively protect the DEN-induced inflammation.
Conclusion
The major goal of this study was to identify the hepatoprotective effects of White-jade, Golden-silk and Light-green strain SMSP because various silkworm byproducts have been shown to several health benefits. Pretreatment with SMSP recovered liver injury and reduced necrotic and histopathological changes, the activities of ALP, AST and ALT induced by DEN in the liver. SMSP also attenuated initial liver injury induced by DEN insult by suppressing the elevation of pro-inflammatory enzymes COX-2 and iNOS. Conclusively, SMSP may have a protective effect on acute liver damage by decreasing hepatotoxicity and inflammatory response in DEN-treated mice. In this study, we compared three strains of silkworm and White-jade SMSP showed the most effective hepatoprotection against DEN treatment. Because HCC occurs in the tissues with inflammation, clearly provokes local hepatic and systemic inflammatory responses, intake of SMSP might be a useful strategy for the chemoprevention of HCC.